Abstract
Quenching of the fluorescence of Trp residues in a membrane protein by lipids with bromine-containing fatty acyl chains provides a powerful technique for measuring lipid-protein binding constants. Single Trp residues have been placed on the periplasmic and cytoplasmic sides of the mechanosensitive channel of large conductance MscL from Mycobacterium tuberculosis to measure, separately, lipid binding constants on the two faces of MscL. The chain-length dependence of lipid binding was found to be different on the two sides of MscL, the chain-length dependence being more marked on the cytoplasmic than on the periplasmic side. To determine if lipid binding constants are affected by the properties of the lipid molecules not in direct contact with MscL (the bulk lipid), the amount of bulk lipid present in the system was varied. The binding constant of the short-chain phospholipid didodecylphosphatidylcholine was found to be independent of the molar ratio of lipid/MscL pentamer over the range 500:1–50:1, suggesting that lipid binding constants are determined largely by the properties of the lipid molecules interacting directly with MscL. These results point to a model in which lipid molecules located on the transmembrane surface of a membrane protein (the annular lipid molecules), by playing a dominant role in the interaction between a membrane protein and the surrounding lipid bilayer, could effectively buffer the membrane protein from changes in the properties of the bulk lipid bilayer.
INTRODUCTION
The lipid bilayer component of a biological membrane acts both as a semipermeable barrier and as a support for the intrinsic membrane proteins. Proper functioning of an intrinsic membrane protein requires a lipid bilayer of the correct composition, since the lipid bilayer provides part of the environment within which the protein has to function. In some cases, this proper function requires the presence of a particular species of lipid molecule, binding to a specific site on a membrane protein (1–4). A clear example is provided by the homotetrameric potassium channel KcsA, where a lipid binding site with specificity for anionic lipid is resolved in the crystal structure at each monomer-monomer interface, with occupation of this site by anionic lipid being necessary for channel opening (5,6). However, most lipid molecules do not interact with membrane proteins in this way. Crystal structures of membrane proteins generally contain either no resolved lipid molecules or a small number, bound between transmembrane α-helices, like those in KcsA (1). In the few cases where a large number of lipid molecules are resolved bound to the transmembrane surface of a membrane protein, as in the cases of bacteriorhodopsin and aquaporin (7,8), this is because the protein molecules are separated in crystalline arrays by just a single layer of lipid molecules, so that the resolved lipid molecules are trapped in the spaces between the protein molecules. In general, lipid molecules bind to the transmembrane surface of a membrane protein with rather low specificity, with individual lipid molecules exchanging rapidly between the protein surface and the bulk lipid bilayer, the lack of order for the lipid molecules explaining why they are usually not resolved in crystal structures of membrane proteins (1–4,9–11). These experimental observations are consistent with the results of molecular dynamics simulations that show that, although lipid molecules next to a protein molecule are perturbed by the presence of the protein molecule whereas the bulk lipid molecules not interacting with the protein are not, the range of interaction energies between bound lipids and the protein is very broad, with the interaction energies fluctuating widely, so that there is no deep energy well into which the lipid falls to give a single favored conformation; the lipid molecules are not frozen in a single long-lived conformation on the protein surface (12). These results suggest that most of the lipid molecules that interact with a membrane protein do so like a typical solvent molecule, allowing parallels to be drawn between the solvation of a membrane protein by lipid and the solvation of a water soluble protein by water.
Studies of the solvation of water soluble proteins by water have emphasized the importance of solvation in defining the structure and function of such proteins (13). Although some water molecules are bound in cavities on the protein surface, others interact less specifically with rather featureless regions of the surface. Water molecules in the hydration shell around a protein have properties that differ significantly from those of the bulk water, the effects of the protein extending only to one or two shells of water molecules around the protein (13,14). Effects of water on the structure and function of water soluble proteins have generally been interpreted in terms of the interactions between the protein and its surrounding hydration shell, rather than in terms of the material properties of bulk water (13). In contrast, a wide range of experiments with membrane proteins have been interpreted in terms of models in which changes in membrane protein structure and function are related to changes in the material properties of the bulk lipid bilayer (for reviews see Lee (2) and McIntosh and Simon (15)). For example, effects of hydrophobic mismatch between a lipid bilayer and a membrane protein have been considered in terms of models of this general type (16–19).
The simplest model for a membrane protein in a lipid bilayer is one where the membrane protein has a smooth, featureless surface and the lipid bilayer is an elastic continuum extending right up to the surface. When the hydrophobic thickness of the protein is less than that of the undistorted lipid bilayer, hydrophobic matching can be achieved by stretching the lipid bilayer around the protein and, conversely, when the hydrophobic thickness of the protein is greater than that of the undistorted lipid bilayer, hydrophobic matching can be achieved by compressing the lipid bilayer around the protein (Fig. 1). The work required to stretch or compress the lipid bilayer can be calculated in terms of mechanical properties of the undistorted lipid bilayer such as the isothermal area expansion/compression modulus, the bilayer bending modulus, and the spontaneous radius of curvature of the bilayer (16–19). Of course, real membrane proteins do not have smooth featureless surfaces; the transmembrane surface of a multihelix membrane protein is rough, containing cavities of various shapes and sizes, ranging from small gaps to large crevices. As described above, the lipid molecules in contact with the protein surface, commonly referred to as the boundary or annular lipids, are perturbed by the protein, and so will have material properties different from those of the lipid molecules not in contact with the protein surface, referred to as the bulk lipids. The question then arises as to the relative importance of the lipid annulus and the bulk lipid for membrane protein structure and function. In particular, are the physical properties of the bulk lipid bilayer transmitted via the annular lipids to a membrane protein or is a membrane protein responsive only to the properties of the annular lipids?
FIGURE 1.
Distortion of a lipid bilayer to achieve hydrophobic matching with a membrane protein. The figure illustrates the stretching (A) or compression (B) of a lipid bilayer around a membrane protein required for hydrophobic matching when the hydrophobic thickness of the lipid bilayer (dl) is less (A) or greater (B) than that of the membrane protein (dp).
Lipid-protein interactions can be studied in functional terms, observing the effect of lipid structure on protein function. However, membrane protein function generally involves a series of conformational changes in the protein and lipid structure can affect differently the rates of the different steps in the reaction cycle, as established, for example, for the Ca2+-ATPase of sarcoplasmic reticulum (20). We therefore decided to study effects of lipid structure on a simple thermodynamic parameter, the binding constant describing lipid binding to a membrane protein.
Fluorescence quenching studies with phospholipids containing brominated fatty acyl chains provide a powerful technique for probing lipid-protein interactions (21–25). Quenching of Trp fluorescence by brominated phospholipids is short range so that only a brominated lipid molecule bound close to a Trp residue can quench its fluorescence (26). This means that the level of quenching of the Trp fluorescence of a membrane protein reconstituted into a mixture of brominated and nonbrominated lipids depends on the binding constant of the protein for the brominated lipid relative to that of the nonbrominated lipid (26). The technique has been applied to the mechanosensitive channel of large conductance (MscL) from Mycobacterium tuberculosis (25,26). The wild-type protein contains no Trp residues, allowing the introduction of single Trp residues into areas of interest on the protein. In the mutant F80W a Trp residue is located in the middle of the lipid bilayer where its fluorescence will be quenched by brominated lipid molecules in either monolayer of the lipid bilayer (Fig. 2). In mutants L69W and Y87W, Trp residues are located on the periplasmic and cytoplasmic sides of MscL, respectively, and fluorescence will only be quenched by brominated lipid molecules on the corresponding sides of the lipid bilayer (Fig. 2).
FIGURE 2.
The structure of the MscL pentamer showing the positions of the three residues L69, F80, and Y87 mutated to Trp. For clarity, residues are shown in only one of the subunits of the homopentameric structure. The horizontal lines show the approximate positions of the glycerol backbone regions on the two sides of the membrane. Coordinates from Chang et al. (35).
In previous studies we have shown that lipid binding constants for MscL vary with fatty acyl chain length, strongest binding being observed when the fatty acyl chain length is that giving a bilayer whose hydrophobic thickness matches the hydrophobic thickness of MscL (26,27). If the effects of lipid chain length on the strength of lipid binding to MscL depend solely on the cost of distorting the lipid bilayer around MscL to achieve hydrophobic matching, then effects of chain length on lipid binding would be expected to be the same on the cytoplasmic and periplasmic sides of MscL in a symmetric lipid bilayer. Here we show that, in fact, effects of lipid chain length on the strength of lipid binding are different on the two sides of the membrane. Further, if the properties of the bulk lipid component of a membrane had an important influence on lipid interactions with a membrane protein then lipid binding constants might be expected to change as the molar ratio of lipid to protein in a membrane is decreased, decreasing the amount of bulk lipid present in the system. We show that the strength of binding of a short-chain phospholipid to MscL is unaffected by changes in the amount of bulk lipid present in the system.
EXPERIMENTAL PROCEDURES
Dimyristoleoylphosphatidylcholine (di(C14:1)PC), dipalmitoleoylphosphatidylcholine (di(C16:1)PC), dioleoylphosphatidylcholine (di(C18:1)PC), dieicosenoylphosphatidylcholine (di(C20:1)PC), dierucoylphosphatidylcholine (di(C22:1)PC), and dinervonylphosphatidylcholine (di(C24:1)PC) were obtained from Avanti Polar Lipids (Alabaster, AL). Didodecylphosphatidylcholine (di(C12:0)PC) was obtained from Sigma (St. Louis, MO). Di(9,10-dibromomyristoyl)phosphatidylcholine (di(Br2C14:0)PC), di(9,10-dibromopalmitoyl)phosphatidylcholine (di(Br2C16:0)PC), di(9,10-dibromostearoyl)phosphatidylcholine (di(Br2C18:0)PC), di(11,12-dibromoeicosanoyl)phosphatidylcholine (di(Br2C20:0)PC), di(13,14-dibromodocosanoyl)phosphatidylcholine (di(Br2C22:0)PC), and di(15,16-dibromotetracosanoyl)phosphatidylcholine (di(Br2C24:0)PC) were prepared as described (21). A plasmid containing the M. tuberculosis mscL gene with a poly-His epitope at the N-terminus was the generous gift of Professor D. C. Rees (California Institute of Technology, Pasadena, CA). Site-directed mutagenesis was performed using the Quick-change protocol from Stratagene (La Jolla, CA). Following polymerase chain reaction mutagenesis the native methylated DNA templates were digested with DpnI (Promega, Madison, WI) for 2 h at 37°C. The mutations were confirmed by DNA sequencing. Escherichia coli BL21(λDE3)pLysS transformants carrying the pET-19b plasmid (Novagen, Madison, WI) with the mscL gene were generally grown in 6 L Luria broth to mid-log phase (OD600 = 0.6) and then induced for 3 h in the presence of isopropyl-β-d-thiogalactopyranoside (1.0 mM). MscL was purified as described by Powl et al. (26) and stored at −80°C until use.
Fluorescence measurements
Purified MscL was reconstituted into lipid bilayers by mixing lipid and MscL in cholate followed by dilution of 250 μl of the detergent-lipid-protein mixture into 2.75 ml buffer (20 mM Hepes, 100 mM KCl, 1 mM EGTA, at pH 7.2) to decrease the concentration of cholate below its critical micelle concentration, as described in Powl et al. (26). The final protein concentration was 1 μM, based on a molecular weight of 93,000 for the MscL pentamer, and the molar ratio of lipid/MscL pentamer was 500:1. Fluorescence was recorded on an SLM 8000C fluorimeter (Urbana, IL) with excitation at 280 nm, at 25°C. Fluorescence emission spectra were corrected for light scatter by subtraction of a blank consisting of lipid alone in buffer. The quoted values for fluorescence emission intensity are the averages of duplicate measurements from two separate reconstitutions.
Analysis of fluorescence quenching results
Quenching of Trp fluorescence in a mixture of a brominated phospholipid with the corresponding nonbrominated phospholipid was fitted to the following equation to give the value of n, the number of lipid binding sites on MscL from which the fluorescence of the Trp residue can be quenched (21,28).
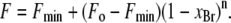 |
(1) |
Here Fo and Fmin are the fluorescence intensities for MscL in nonbrominated and in brominated lipid, respectively, and F is the fluorescence intensity in the phospholipid mixture when the mol fraction of brominated lipid is xBr. For the mutants L69W, F80W, and Y87W n was found to be independent of fatty acyl chain lengths and to have values of 2.1 ± 0.15, 2.5 ± 0.1, and 1.9 ± 0.15, respectively.
In a mixture of a nonbrominated lipid A and a brominated lipid B, an equilibrium will be established at each lattice site:
 |
where PA and PB are protein bound to lipids A and B, respectively, and the binding constant for B relative to A is given by
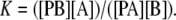 |
(2) |
Fluorescence quenching in the mixture is described by the equation
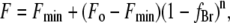 |
(3) |
where fBr, the fraction of sites on MscL occupied by brominated lipid, is given by
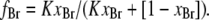 |
(4) |
Relative binding constants were determined from fluorescence quenching plots of the MscL mutants in mixtures of di(Br2C18:0)PC with di(C12:0)PC, di(C14:1)PC, di(C16:1)PC, di(C18:1)PC, di(C20:1)PC, di(C22:1)PC, or di(C24:1)PC and from mixtures of di(C18:1)PC with the corresponding brominated lipid. Equations 1 and 3 were fitted to the experimental data using the nonlinear least-squares routine in the SigmaPlot package (SPSS, Chicago, IL).
Gradient centrifugation
Gradient centrifugation was used to characterize the reconstituted preparation. For the sample containing a 100:1 molar ratio of lipid/MscL pentamer, di(C18:1)PC (9.12 μmols) was mixed with rhodamine-labeled phosphatidylethanolamine (0.48 μmols) in chloroform and dried onto the walls of a glass vial. The lipid mixture was resuspended in buffer (1.6 ml; 20 mM Hepes, 100 mM KCl, 1 mM EGTA, pH 7.2) containing 15 mM cholate and sonicated to clarity in a sonication bath. F80W (8.93 mg) was added and the mixture incubated at 25°C for 15 min. The sample was then dialyzed at 4°C against two lots of buffer (500 ml; 20 mM Hepes, 100 mM KCl, 1 mM EGTA, pH 7.2) for a total of 16 h. Samples of dialysate (1.5 ml) were then loaded onto sucrose gradients containing the following solutions of sucrose (w/v) in the above buffer: 10, 20, 30, 40, 50, and 60%; the 60% sucrose solution also contained 0.05% (w/v) Triton X-100. Samples were spun at 80,000 × g for 18 h at 4°C and then 1.5 ml fractions were collected from the gradients and analyzed for lipid and protein by, respectively, absorbance at 570 nm and BioRad (Hercules, CA) protein assay. Similar protocols were used for samples with a lower molar ratio of lipid/protein except that for samples at 30:1 and 20:1 molar ratios of lipid/MscL pentamer a lipid mixture containing 20 mol % of rhodamine-labeled phosphatidylethanolamine was used to allow for accurate determination of lipid amounts.
RESULTS
Relative binding constants on the two sides of MscL
The MscL mutants L69W and Y87W contain Trp residues on what would be the periplasmic and cytoplasmic sides of MscL, respectively, in the native membrane (Fig. 2). Measuring fluorescence quenching of L69W and Y87W in mixtures of brominated and nonbrominated phospholipid therefore enables us to measure lipid binding constants for the periplasmic and cytoplasmic sides of MscL, respectively, even though in these experiments MscL is reconstituted randomly into a symmetric lipid bilayer.
Fluorescence quenching curves for L69W in mixtures of di(Br2C18:0)PC and phosphatidylcholines of chain lengths of C12, C16, C18, and C22 are shown in Fig. 3 A. The curves are all very similar, showing that all these lipids bind to the periplasmic side of MscL with a similar affinity. In contrast, for Y87W fluorescence quenching is more marked in mixtures of di(C12:0)PC and di(Br2C18:0)PC at intermediate mol fractions of di(Br2C18:0)PC than in mixtures of di(C16:1)PC and di(Br2C18:0)PC, showing that di(C12:0)PC binds to the cytoplasmic side of MscL with an affinity less than that for di(C16:1)PC (Fig. 3 B). Similarly, fluorescence quenching for Y87W is more marked in mixtures of di(C22:1)PC and di(Br2C18:0)PC at intermediate mol fractions of di(Br2C18:0)PC than in mixtures of di(C16:1)PC and di(Br2C18:0)PC, showing that di(C22:1)PC also binds to the cytoplasmic side of MscL with an affinity less than that for di(C16:1)PC. Quenching profiles for other chain-length lipids were intermediate between those shown in Fig. 3 B. Data were fitted to Eq. 3, giving the relative binding constants plotted in Fig. 4. Lipid binding constants clearly vary more with chain length on the cytoplasmic than on the periplasmic side of MscL.
FIGURE 3.
Quenching of fluorescence by di(Br2C18:0)PC. L69W (A) and Y87W (B) were reconstituted into bilayers containing mixtures of di(Br2C18:0)PC and (▵) di(C12:0)PC, (○) di(C16:1)PC, (∇) di(C18:1)PC, and (□) di(C22:1)PC. The solid lines show best fits to Eq. 3 giving the relative binding constants plotted in Fig. 4. Experimental values of fluorescence intensity are averages of duplicate measurements from two separate reconstitutions.
FIGURE 4.
Relative lipid binding constants for the two faces of MscL. Lipid binding constants for a series of phosphatidylcholines relative to that for di(C18:1)PC were determined from the data plotted in Fig. 3 for the periplasmic face of MscL, using L69W (□), and for the cytoplasmic face, using Y87W (○), with di(Br2C18:0)PC as the quenching lipid. All the lipids contained monounsaturated fatty acyl chains except for di(C12:0)PC.
A potential complication in analyzing the data in experiments with mixtures of two lipids with different chain lengths is that varying the relative proportions of the two lipids will change the thickness of the bulk lipid bilayer and this could change the distance of separation between the Trp residue in MscL and the bromines in the brominated phospholipid, so affecting the efficiency of quenching. However, this appears not to be the case. Table 1 lists values of fluorescence quenching for L69W and Y87W in bilayers of a series of brominated phospholipids of chain lengths between C14 and C24. For chain lengths between C14 and C18, the level of fluorescence quenching changes only vary slightly with changing chain length, and thus is almost independent of bilayer thickness. Although levels of fluorescence quenching are different for di(Br2C18:0)PC and the longer-chain brominated phospholipids, this can be attributed to changes in the positions of the bromines within the longer chains (Table 1); changing the positions of the bromines within a lipid chain has been shown to affect the efficiency of quenching of the Trp residue in a transmembrane α-helix (29).
TABLE 1.
Fluorescence quenching as a function of phospholipid chain length
| Position of bromines within the chain |
F/Fo
|
||
|---|---|---|---|
| Chain length | L69W | Y87W | |
| C14 | 9,10 | 0.36 ± 0.04 | 0.27 ± 0.02 |
| C16 | 9,10 | 0.42 ± 0.01 | 0.29 ± 0.01 |
| C18 | 9,10 | 0.43 ± 0.01 | 0.30 ± 0.02 |
| C20 | 11,12 | 0.57 ± 0.01 | 0.33 ± 0.01 |
| C22 | 13,14 | 0.64 ± 0.01 | 0.41 ± 0.02 |
| C24 | 15,16 | 0.71 ± 0.01 | 0.48 ± 0.02 |
F and Fo correspond to fluorescence intensities for L69W or Y87W in bilayers of brominated or nonbrominated phosphatidylcholines, respectively, of the given fatty acyl chain length. The molar ratio of lipid/MscL pentamer was 500:1. Measurements are the averages of duplicate measurements from two separate reconstitutions.
The appropriateness of the analysis of the fluorescence data was checked by determining fluorescence quenching curves for the reverse experiment, in which MscL was reconstituted in mixtures of di(C18:1)PC with brominated phospholipids with chain lengths between C14 and C24 (Fig. 5). If changing bilayer thickness as such had any significant effect on the efficiency of fluorescence quenching, then the results of the two sets of experiments would be different. For example, in the pair of experiments with mixtures of lipids with C14 and C18 chain lengths, adding di(C14:1)PC to di(Br2C18:0)PC would result in a thinning of the bilayer whereas adding di(C18:1)PC to di(Br2C14:0)PC would result in a thickening of the bilayer. However, the relative lipid binding constants obtained from experiments with mixtures of di(C18:1)PC and brominated phospholipids (Fig. 6) agree within experimental error with those obtained from the experiments with di(Br2C18:0)PC (Fig. 4). In particular, the more marked chain-length dependence of binding for Y87W than for L69W is again apparent.
FIGURE 5.
Quenching of fluorescence by brominated lipids in mixtures with di(C18:1)PC. L69W (A) and Y87W (B) were reconstituted into bilayers containing mixtures of di(C18:1)PC and (•) di(Br2C14:0)PC, (○) di(Br2C18:0)PC, (▪) di(Br2C22:0)PC, and (□) di(Br2C24:1)PC. The solid lines show best fits to Eq. 3 giving the relative binding constants plotted in Fig. 6. Experimental values of fluorescence intensity are averages of duplicate measurements from two separate reconstitutions.
FIGURE 6.
Relative lipid binding constants for the two faces of MscL, determined using brominated lipids of different chain lengths. Lipid binding constants for a series of phosphatidylcholines relative to that for di(C18:1)PC were determined from the data plotted in Fig. 5, for the periplasmic face of MscL, using L69W (□), and for the cytoplasmic face, using Y87W (○), from mixtures of di(C18:1)PC and the corresponding brominated lipid.
An interesting comparison can be made with the chain-length dependence of lipid binding to MscL previously published using the mutant F80W (26). In F80W the Trp residue is located toward the center of the lipid bilayer (Fig. 2), where it will be quenched by brominated lipids bound on either side of the protein. As shown in Fig. 7, relative lipid binding constants measured using F80W agree well with the average of the lipid binding constants on the periplasmic and cytoplasmic sides of MscL. In previous studies with the F93W mutant of MscL from E. coli (26) we established a more marked chain-length dependence of lipid binding than that measured with the F80W mutant of MscL from M. tuberculosis. It is now clear that these differences do not reflect differences between MscL from E. coli and M. tuberculosis but rather reflect the fact that the Trp residue in the F93W mutant of MscL from E. coli is located on the cytoplasmic side of the membrane.
FIGURE 7.
Average relative lipid binding constants for MscL. The average of the lipid binding constants determined for the periplasmic and cytoplasmic faces of MscL (○) are compared to those determined using F80W (□). The data for F80W is taken from Powl et al. (26).
Minimum amount of lipid required for incorporation of MscL into a lipid bilayer
Studies with simple hydrophobic Trp-containing peptides have shown that fluorescence intensities are high when the peptides are incorporated into lipid bilayers but are low for aggregates of the peptides in water (30). Plots of fluorescence intensity versus molar ratio of lipid/peptide define the minimum amount of lipid required for full incorporation of the peptides into lipid bilayers (30).
F80W was reconstituted into bilayers of di(C18:1)PC at various molar ratios of di(C18:1)PC/MscL. A plot of Trp fluorescence intensity as a function of molar ratio suggests that a minimum of ∼40–50 lipid molecules per MscL pentamer are required for complete incorporation into the bilayer (Fig. 8). A similar estimate is suggested from experiments measuring fluorescence quenching in bilayers of di(Br2C18:0)PC where incorporation of MscL into the bilayer leads to fluorescence quenching (Fig. 8).
FIGURE 8.
Effect of molar ratio of lipid/MscL pentamer on bilayer incorporation of MscL. Fractional changes in fluorescence intensity [(F − Fw)/(FO − Fw)] are plotted as a function of molar ratio of phospholipid/MscL pentamer in di(C18:1)PC (left axis; ○) or di(Br2C18:0)PC (right axis; □). FO is the fluorescence intensity at a 300:1 molar ratio of lipid/MscL pentamer and FW is the fluorescence intensity in the absence of lipid. Measurements are the averages of duplicate measurements from two separate reconstitutions.
Effect of lipid/protein molar ratio on lipid binding constants
To study the effect of lipid/protein molar ratio on lipid binding constants, F80W was reconstituted into lipid bilayers from detergent solutions containing different molar ratios of lipid to protein. Sucrose gradient centrifugation was used to confirm homogeneous mixing of lipid and protein in these mixtures. With a discontinuous gradient from 10 to 60 mol % sucrose, lipid without MscL is found at the top of the gradient (26). As shown in Fig. 9, when lipid and MscL are mixed at a molar ratio of lipid/MscL pentamer of 100:1 or 50:1 the lipid colocalizes with MscL toward the center of the gradients. When reconstituted at a molar ratio of 30:1 or 20:1, the majority of the lipid and protein again colocalize, but now toward the bottom of the gradient, due to the greater density of the reconstituted membrane. The results of these experiments therefore show that the mixing of lipid and protein in the reconstituted membranes is largely homogeneous.
FIGURE 9.
Sucrose gradient analysis of the reconstituted MscL. Samples of F80W reconstituted with di(C18:1)PC at various lipid/protein molar ratios were separated on a discontinuous sucrose gradient from 10 to 60% sucrose; 1.5 ml fractions were taken and analyzed for lipid (•, ▾) and MscL (○, ∇). Molar ratios lipid/MscL pentamer are: (A) (○, •) 100:1 and (∇, ▾) 30:1; (B) (○,•) 50:1 and (∇, ▾) 20:1.
To determine the effect of lipid/protein ratio on lipid binding constants, F80W was reconstituted into mixtures of di(C12:0)PC and di(Br2C18:0)PC and fluorescence intensities were recorded as a function of the mol fraction of di(Br2C18:0)PC in the mixture, at molar ratios of total phospholipid/MscL pentamer between 500:1 and 20:1. Fluorescence quenching curves at molar ratios of lipid/MscL pentamer greater than 40:1 were superimposable but quenching at a molar ratio of lipid/MscL pentamer of 20:1 was less extensive and the quenching curve was shallower than that observed at higher molar ratios of lipid (Fig. 10). Binding constants for di(C12:0)PC relative to di(C18:1)PC derived from these data are listed in Table 2 as a function of the molar ratio of total phospholipid/MscL pentamer. When the molar ratio of lipid/MscL pentamer is less than that required to form a complete annular shell around the protein, all the lipid will be annular lipid and the fraction of annular sites occupied by brominated lipid will be equal to the fraction of brominated lipid in the total lipid mixture; the fluorescence analysis should then return a value for K of 1, as observed experimentally when the molar ratio of lipid/MscL pentamer is 20:1 (Table 2). At higher molar ratios of lipid/MscL pentamer the binding constant for di(C12:0)PC relative to di(C18:1)PC is ∼0.7 and does not change significantly over the molar ratio range 50:1–500:1 (Table 2).
FIGURE 10.
Effect of molar ratio of lipid/MscL pentamer on fluorescence quenching in mixtures of di(C12:0)PC and di(Br2C18:0)PC. F80W was reconstituted into bilayers containing mixtures of di(C12:0)PC and di(Br2C18:0)PC at molar ratios of total phospholipid/MScL pentamer of: (▵) 100:1; (○) 50:1; (∇) 40:1; (□) 20:1. The solid lines show best fits to Eq. 3 giving the relative binding constants listed in Table 2. Measurements are the averages of duplicate measurements from two separate reconstitutions.
TABLE 2.
Effect of lipid/protein molar ratio on the relative lipid binding constant for di(C12:0)PC
| Molar ratio lipid/MscL pentamer | Binding constant di(C12:0)PC relative to di(C18:1)PC |
|---|---|
| 500:1 | 0.71 ± 0.06 |
| 100:1 | 0.67 ± 0.06 |
| 50:1 | 0.69 ± 0.08 |
| 40:1 | 0.78 ± 0.08 |
| 20:1 | 0.94 ± 0.09 |
Relative lipid binding constants for F80W were determined at pH 7.2 from plots of fluorescence quenching in mixtures of di(C12:0)PC and di(Br2C18:0)PC, with a value for n, the number of lipid binding sites from which the Trp fluorescence can be quenched, of 2.5.
DISCUSSION
Hydrophobic matching on the two sides of a membrane
The efficiency of hydrophobic matching between a membrane protein and the surrounding lipid bilayer is high (23). Any mismatch between the hydrophobic thicknesses of an undistorted membrane protein and an undistorted lipid bilayer could, in principle, be overcome by distorting the lipid bilayer with no changes in the protein, by distorting the protein with no changes in the lipid bilayer, or by distorting both the lipid bilayer and the protein. These distortions will be reflected in the values of the binding constants describing the strength of lipid binding to the protein; the binding constant of a lipid whose binding involves no distortion will be greater than that of a lipid whose binding requires distortion of either the lipid bilayer or the protein (26).
If hydrophobic matching is achieved solely by distortion of the lipid bilayer, and if the protein surface is assumed to be smooth and featureless (Fig. 1), then the effects of lipid chain length on lipid binding would be expected to be the same on the two sides of a symmetric membrane. Using the mutants L69W and Y87W (Fig. 2) it is possible to measure, separately, relative lipid binding constants on the periplasmic and cytoplasmic sides of MscL. As shown in Figs. 4 and 6, the chain-length dependencies of lipid binding detected by the mutants L69W and Y87W are distinctly different. These results suggests either that the chain-length dependence of binding at each lipid binding site on MscL is different, or that the chain-length dependence of lipid binding is different on the two sides of the membrane. Experiments changing the brominated lipid species suggest that the latter is more likely. The circumference of the transmembrane domain of the MscL pentamer can be estimated from the crystal structure (35) to be ∼135 Å, which, together with a diameter for a lipid molecule of 9.4 Å, suggests that ∼30 lipid molecules are required to form a complete bilayer shell around the protein, giving approximately three lipid binding sites per monomer on each side of the membrane. The number of lipid binding sites from which the fluorescence of a Trp residue in MscL can be quenched (n in Eq. 1) is ∼2. If the relative lipid binding constants are the same at these two sites, the same binding constant for lipid X relative to di(C18:1)PC will be obtained from experiments with mixtures of di(Br2C18:1)PC and lipid X, and from experiments with mixtures of di(C18:1)PC and brominated lipid X. However, if the two sites have different relative affinities for lipid X and di(C18:1)PC, the results obtained from the two sets of experiments will be different (25). For example, if the first site has a higher affinity for lipid X than for di(C18:1)PC and the second site has a higher affinity for di(C18:1)PC than for lipid X, experiments with brominated lipid X will be dominated by binding of lipid X with high affinity to the first site, returning a high binding constant for lipid X relative to di(C18:1)PC, whereas experiments with di(Br2C18:1)PC will be dominated by binding of di(Br2C18:1)PC with high affinity to the second site, returning a low binding constant for lipid X relative to di(C18:1)PC. In fact binding constants returned from the two sets of experiments agree within experimental error (Figs. 4 and 6) showing that the chain-length dependencies of binding at the two sites around the Trp residue in L69W are the same, as are those around the Trp residue in Y87W. A similar result was obtained previously for the sites around the Trp residue in F80W (26). We conclude therefore that it is most likely that the different chain-length dependencies reported by L69W and Y87W reflect different chain-length dependencies of binding on the two sides of the membrane, with the chain-length dependence being more marked on the cytoplasmic than on the periplasmic side. This result suggests that a more complex model may be required to describe lipid-protein interactions than that provided by current theoretical models.
The chain-length dependencies of lipid binding to a number of α-helical membrane proteins have been shown to be less marked than expected if hydrophobic matching relied solely on distortion of the lipid bilayer around a rigid protein molecule (26). Further, it has been shown that the chain-length dependencies of lipid binding to α-helical membrane proteins are less marked than that for binding to the β-barrel protein OmpF (21–23,26). Given the relative ease of tilting of an α-helix in a lipid bilayer (30–32), these results suggest that hydrophobic matching for α-helical proteins could involve tilting of transmembrane α-helices as well as distortion of the lipid bilayer. Distortion of α-helical membrane proteins as part of the process of hydrophobic matching would be consistent with the known effect of fatty acyl chain length on membrane protein function (2). The data in Figs. 4 and 6 would then suggest that MscL distorts more easily on the periplasmic side than on the cytoplasmic side. Different responses to hydrophobic matching on the two sides of the membrane implies that hydrophobic matching for MscL involves bending of transmembrane α-helices because a simple tilting of a rigid helix would result in the same changes in hydrophobic thickness on the two sides of the protein. A molecular dynamics simulation of MscL in thin lipid bilayers detected bending in the second transmembrane α-helix (33) and a normal mode analysis of MscL suggested that magnitudes of motion increased from the cytoplasmic to the periplasmic end of the helix (34).
The observation that the lipid chain length giving strongest binding to MscL is approximately C16 (Figs. 4 and 6) is consistent with the hydrophobic thickness of MscL of 26 Å estimated from Trp scanning fluorescence studies since a bilayer of di(C16:1)PC has a hydrophobic thickness of ∼24 Å (27).
The relative importance of annular and bulk lipid
To determine whether lipid-protein interactions are affected by the presence of bulk lipid in the membrane, mediated via the annular lipid molecules, we studied the effect of reducing the amount of bulk lipid. As shown in Table 2, binding constants for di(C12:0)PC remained constant on decreasing the molar ratio of lipid/MscL pentamer from 500:1 to 50:1. Although, as described above, the relative dimensions of the lipid and MscL molecules suggest that ∼30 lipid molecules are required to form a complete bilayer shell around the protein, if the distribution of lipid molecules between annular and bulk lipid not in contact with protein is close to random, then the molar ratio of lipid/MscL pentamer required to give a complete annular shell around each MscL pentamer would be somewhat >30:1 (see East et al. (11)). This would be consistent with the data in Fig. 8 showing that complete incorporation of MscL into a lipid bilayer requires a molar ratio of lipid/MscL pentamer of ∼50:1. Over the lipid/MscL pentamer molar ratio range of 500:1–50:1 the number of lipid shells around each MscL pentamer will decrease from ∼7 to ∼1.5. With further decreases in the molar ratio of lipid/MscL pentamer the value of the relative binding constant for di(C12:0)PC increases toward 1, as it must do, since when all the lipid in the system is annular lipid, the proportion of lipid in the annulus that is di(C12:0)PC will be equal to the proportion of the total lipid in the system that is di(C12:0)PC.
The constancy of the binding constant for di(C12:0)PC over the lipid/MscL pentamer molar ratio range of 500:1–50:1 suggest either that the properties of the bulk lipid bilayer do not significantly affect the strength of binding of di(C12:0)PC to MscL or that the relevant properties of the bulk and annular lipids are the same. Arguing against the latter possibility is much evidence that suggests that the annular lipid molecules have properties that differ significantly from those of the bulk lipid molecules. For example, ESR studies suggest that the rotational mobilities of annular lipid molecules are impeded by interaction with the protein surface (36). Similarly, molecular dynamics simulations suggest that fatty acyl chain and lipid headgroup conformations and mobilities for the annular lipid molecules differ from those of bulk lipid molecules, and that the effects of a membrane protein on the properties of the lipid molecules in a membrane are largely restricted to the annular lipids, the properties of the bulk lipids being largely unaffected (reviewed in Lee (37)). Thus the mechanical properties of a lipid molecule on the surface of a membrane protein are unlikely to be the same as those of a bulk lipid molecule. It has been shown, for example, that the presence of cholesterol has a very large effect on the elastic properties of a lipid bilayer that can be understood in terms of different elastic properties for free lipid molecules and for lipid-cholesterol complexes (38).
These results would then suggest that the strength of the lipid-protein interaction is dominated by direct interactions with the annular lipids, any effects of the bulk lipid bilayer on the interaction being small. If these results can be extended to effects on membrane protein function then they would suggest that the lipid annulus, by playing a dominant role in the interaction between a membrane protein and the surrounding lipid bilayer, will effectively buffer the membrane protein from changes in the properties of the bulk lipid bilayer.
Acknowledgments
We thank Professor D. C. Rees for the gift of the MscL construct.
This work was financially supported by the Wellcome Trust.
Editor: Antoinette Killian.
References
- 1.Lee, A. G. 2003. Lipid-protein interactions in biological membranes: a structural perspective. Biochim. Biophys. Acta. 1612:1–40. [DOI] [PubMed] [Google Scholar]
- 2.Lee, A. G. 2004. How lipids affect the activities of integral membrane proteins. Biochim. Biophys. Acta. 1666:62–87. [DOI] [PubMed] [Google Scholar]
- 3.Palsdottir, H., and C. Hunter. 2004. Lipids in membrane protein structures. Biochim. Biophys. Acta. 1666:2–18. [DOI] [PubMed] [Google Scholar]
- 4.Wiener, M. 2005. A census of ordered lipids and detergents in x-ray crystal structures of integral membrane proteins. In Protein-Lipid Interactions: From Membrane Domains to Cellular Networks. L. K. Tamm, editors. Wiley-VCH, Weinheim, Germany. 97–118.
- 5.Valiyaveetil, F. I., Y. Zhou, and R. MacKinnon. 2002. Lipids in the structure, folding and function of the KcsA K+ channel. Biochemistry. 41:10771–10777. [DOI] [PubMed] [Google Scholar]
- 6.Marius, P., S. J. Alvis, J. M. East, and A. G. Lee. 2005. The interfacial lipid binding site on the potassium channel KcsA is specific for anionic phospholipids. Biophys. J. 89:4081–4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luecke, H., B. Schobert, H. T. Richter, J. P. Cartailler, and J. K. Lanyi. 1999. Structure of bacteriorhodopsin at 1.55 angstrom resolution. J. Mol. Biol. 291:899–911. [DOI] [PubMed] [Google Scholar]
- 8.Gonen, T., Y. Cheng, P. Sliz, Y. Hiroaki, Y. Fujiyoshi, S. C. Harrison, and T. Walz. 2005. Lipid-protein interactions in double-layered two-dimensional AQP0 crystals. Nature. 438:633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marsh, D., and T. Pali. 2004. The protein-lipid interface: perspectives from magnetic resonance and crystal structures. Biochim. Biophys. Acta. 1666:118–141. [DOI] [PubMed] [Google Scholar]
- 10.Davoust, J., and J. P. Devaux. 1982. Simulations of electron-spin resonance spectra of spin-labeled fatty acids covalently attached to the boundary of an intrinsic membrane protein—a chemical exchange model. J. Magn. Res. 48:475–494. [Google Scholar]
- 11.East, J. M., D. Melville, and A. G. Lee. 1985. Exchange rates and numbers of annular lipids for the calcium and magnesium ion dependent adenosinetriphosphatase. Biochemistry. 24:2615–2623. [DOI] [PubMed] [Google Scholar]
- 12.Woolf, T. B., and B. Roux. 1996. Structure, energetics, and dynamics of lipid-protein interactions: a molecular dynamics study of the gramicidin A channel in a DMPC bilayer. Proteins. 24:92–114. [DOI] [PubMed] [Google Scholar]
- 13.Raschke, T. M. 2006. Water structure and interactions with protein surfaces. Curr. Opin. Struct. Biol. 16:152–159. [DOI] [PubMed] [Google Scholar]
- 14.Modif, K., E. Liepinsh, G. Otting, and B. Halle. 2004. Dynamics of protein and peptide hydration. J. Amer. Chem. Soc. 126:102–114. [DOI] [PubMed] [Google Scholar]
- 15.McIntosh, T. J., and S. A. Simon. 2006. Roles of bilayer material properties in function and distribution of membrane proteins. Annu. Rev. Biophys. Biomol. Struct. 35:177–198. [DOI] [PubMed] [Google Scholar]
- 16.Mouritsen, O. G., and M. Bloom. 1984. Mattress model of lipid-protein interactions in membranes. Biophys. J. 46:141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fattal, D. R., and A. Ben-Shaul. 1993. A molecular model for lipid-protein interaction in membranes: the role of hydrophobic mismatch. Biophys. J. 65:1795–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen, C., M. Goulian, and O. S. Andersen. 1998. Energetics of inclusion-induced bilayer deformations. Biophys. J. 74:1966–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiggins, P., and R. Phillips. 2005. Membrane-protein interactions in mechanosensitive channels. Biophys. J. 88:880–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, A. G. 1998. How lipids interact with an intrinsic membrane protein: the case of the calcium pump. Biochim. Biophys. Acta. 1376:381–390. [DOI] [PubMed] [Google Scholar]
- 21.East, J. M., and A. G. Lee. 1982. Lipid selectivity of the calcium and magnesium ion dependent adenosinetriphosphatase, studied with fluorescence quenching by a brominated phospholipid. Biochemistry. 21:4144–4151. [DOI] [PubMed] [Google Scholar]
- 22.O'Keeffe, A. H., J. M. East, and A. G. Lee. 2000. Selectivity in lipid binding to the bacterial outer membrane protein OmpF. Biophys. J. 79:2066–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williamson, I. M., S. J. Alvis, J. M. East, and A. G. Lee. 2002. Interactions of phospholipids with the potassium channel KcsA. Biophys. J. 83:2026–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alvis, S. J., I. M. Williamson, J. M. East, and A. G. Lee. 2003. Interactions of anionic phospholipids and phosphatidylethanolamines with the potassium channel KcsA. Biophys. J. 85:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powl, A. M., J. M. East, and A. G. Lee. 2005. Heterogeneity in the binding of lipid molecules to the surface of a membrane protein: hot-spots for anionic lipids on the mechanosensitive channel of large conductance MscL and effects on conformation. Biochemistry. 44:5873–5883. [DOI] [PubMed] [Google Scholar]
- 26.Powl, A. M., J. M. East, and A. G. Lee. 2003. Lipid-protein interactions studied by introduction of a tryptophan residue: the mechanosensitive channel MscL. Biochemistry. 42:14306–14317. [DOI] [PubMed] [Google Scholar]
- 27.Powl, A. M., J. N. Wright, J. M. East, and A. G. Lee. 2005. Identification of the hydrophobic thickness of a membrane protein using fluorescence spectroscopy: studies with the mechanosensitive channel MscL. Biochemistry. 44:5713–5721. [DOI] [PubMed] [Google Scholar]
- 28.London, E., and G. W. Feigenson. 1981. Fluorescence quenching in model membranes. 2. Determination of local lipid environment of the calcium adenosinetriphosphatase from sarcoplasmic reticulum. Biochemistry. 20:1939–1948. [DOI] [PubMed] [Google Scholar]
- 29.Bolen, E. J., and P. W. Holloway. 1990. Quenching of tryptophan fluorescence by brominated phospholipid. Biochemistry. 29:9638–9643. [DOI] [PubMed] [Google Scholar]
- 30.Webb, R. J., J. M. East, R. P. Sharma, and A. G. Lee. 1998. Hydrophobic mismatch and the incorporation of peptides into lipid bilayers: a possible mechanism for retention in the Golgi. Biochemistry. 37:673–679. [DOI] [PubMed] [Google Scholar]
- 31.Park, S. H., and S. J. Opella. 2005. Tilt angle of a trans-membrane helix is determined by hydrophobic mismatch. J. Mol. Biol. 350:310–318. [DOI] [PubMed] [Google Scholar]
- 32.Duong-Ly, K. C., V. Nanda, W. F. DeGrado, and K. P. Howard. 2005. The conformation of the pore region of the M2 proton channel depends on lipid bilayer environment. Protein Sci. 14:856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elmore, D. E., and D. A. Dougherty. 2003. Investigating lipid composition effects on the mechanosensitive channel of large conductance (MscL) using molecular dynamics simulations. Biophys. J. 85:1512–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valadie, H., J. J. Lacapcre, Y. H. Sanejouand, and C. Etchebest. 2003. Dynamical properties of the MscL of Escherichia coli: a normal mode analysis. J. Mol. Biol. 332:657–674. [DOI] [PubMed] [Google Scholar]
- 35.Chang, G., R. H. Spencer, A. T. Lee, M. T. Barclay, and D. C. Rees. 1998. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science. 282:2220–2226. [DOI] [PubMed] [Google Scholar]
- 36.Brotherus, J. K., O. H. Griffith, M. O. Brotherus, P. C. Jost, and J. R. Silvius. 1981. Lipid-protein multiple binding equilibria in membranes. Biochemistry. 20:5261–5267. [DOI] [PubMed] [Google Scholar]
- 37.Lee, A. G. 2005. How lipids and proteins interact in a membrane: a molecular approach. Molecular Biosystems. 1:1–11. [DOI] [PubMed] [Google Scholar]
- 38.Needham, D., and R. S. Nunn. 1990. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys. J. 58:997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]












