Abstract
The widely accepted notion that the benefits of antenatal corticosteroids decline with time to birth may not be correct, argue Simon Gates and Peter Brocklehurst, as the evidence is based on unsound subgroup analyses
The effectiveness of antenatal corticosteroids to prevent neonatal lung disease in women at risk of preterm birth was established by systematic reviews. In addition, subgroup analyses suggested that treatment was most effective in babies born one to seven days after administration. This belief led to widespread use of repeated courses of corticosteroids in women who did not deliver within a week or two of initial treatment. However, the notion that effectiveness declines after seven days may be incorrect, as the analyses that it is based on are unreliable. Here, we discuss the methodological problems of these analyses and their relevance to current randomised controlled trials of repeated versus single courses.
So, what is the evidence?
Babies born before 32 weeks' gestation often have neonatal lung disease, a major cause of neonatal mortality and morbidity—the earlier the birth, the greater the risk. Corticosteroids given to mothers at risk of preterm delivery accelerate fetal lung development, and the effectiveness of this treatment for preventing neonatal lung disease was investigated in a series of randomised controlled trials from the 1970s onwards. Some trials showed a significant benefit of antenatal corticosteroids, but some showed no significant effect. A landmark systematic review1 2 3 resolved the apparent discrepancies in the results and established that this treatment reduced death and respiratory distress syndrome in the babies of these women. A forest plot from this review is used in the Cochrane Collaboration's logo, and the intervention is now used routinely in clinical practice.
Another clinically important question is whether (and how) the effectiveness of antenatal corticosteroids changes with time after administration. This question was investigated by a subgroup analysis in the first randomised controlled trial conducted.4 Women were divided into subgroups on the basis of the interval between treatment and delivery—less than 24 hours, 24-48 hours, two to seven days, and more than seven days. Respiratory distress syndrome was significantly reduced only in babies born two to seven days after the first dose of corticosteroids. The somewhat inconsistent conclusion was that steroids should be given at least 24 hours before delivery to have a noticeable effect on lung function,4 and that effectiveness does not persist for more than a week.5 This conclusion appears to be consistent with the results of laboratory studies,6 although corticosteroids act in several different ways, and how they affect growth and development of the lungs is not certain.7 Subsequent trials and four systematic reviews1 2 3 8 found similar results—a large and statistically significant reduction in respiratory distress syndrome in the subgroup given corticosteroids one to seven days before delivery and a smaller (usually non-significant) effect in the other subgroups (table). This evidence led, in the 1990s, to a widespread practice of repeating treatment in women who did not deliver within seven to 10 days of receiving it.9 10 Courses were often repeated weekly until 34 weeks' gestation, resulting in prolonged exposure of babies to corticosteroids. More recently, use of multiple courses has declined, because of worries about adverse effects of exposure to corticosteroids, especially in the developing brain. At least nine randomised controlled trials have been initiated to determine the efficacy and safety of repeated courses. The studies reported so far have shown no conclusive evidence of short term benefit for repeated courses, but long term follow-up is needed to assess the neurodevelopmental effects of this treatment and fully understand its risks and benefits.11 12 13
Meta-analyses of trials of corticosteroid treatment for preventing neonatal respiratory distress syndrome in women at risk of preterm delivery
| Meta-analysis | Time period subgroup | No of trials | Intervention group | Odds ratio (95% confidence interval) | |
|---|---|---|---|---|---|
| Treatment* | Control* | ||||
| Crowley 19891 | <24 h | 10 | 61/283 | 84/297 | 0.72 (0.49 to 1.06) |
| 24 h to 7 days | 12 | 68/761 | 155/718 | 0.31 (0.23 to 0.42) | |
| >7 days | 6 | 27/365 | 36/372 | 0.62 (0.35 to 1.08) | |
| Crowley 19902 | 24 h to 7 days | Not stated | Not stated | Not stated | 0.31 (0.23 to 0.42) |
| <24 h or >7 days | Not stated | Not stated | Not stated | 0.69 (0.50 to 0.94) | |
| Crowley 19953 | <24 h | 12 | 78/383 | 99/397 | 0.80 (0.56 to 1.15) |
| 24 h to 7 days | 13 | 87/855 | 185/812 | 0.35 (0.26 to 0.46) | |
| >7 days | 7 | 32/379 | 41/384 | 0.63 (0.38 to 1.07) | |
| Roberts and Dalziel 20068 | <24 h | 9 | 68/260 | 74/257 | 0.82 (0.55 to 1.22) |
| 24 h to 7 days | 9 | 57/563 | 126/547 | 0.36 (0.25 to 0.51) | |
| >7 days | 8 | 32/498 | 37/490 | 0.80 (0.48 to 1.33) | |
*Number of events/number of babies.
What are the problems?
Although the notion of an optimal period of administration persists,14 15 certain features of the analyses on which this conclusion is based could make them unreliable and the conclusion unsound. We describe four ways in which misleading results could have arisen.
Arbitrary choice of time period subgroups
The choice of 24 hours and seven days as the cut-off points for determining the subgroups was totally arbitrary. These time points were used by the first trial to be published and have been followed by others, but the reasons for choosing them are not clear. They may have been chosen to maximise the difference between the subgroups, and different cut-off points might have produced different results. If a period of maximum effectiveness does exist, it may not be a plateau between days one and seven.
Babies born at term were all in one subgroup
Most trials recruited women with a gestational age of less than 36 weeks. Almost all babies born at term (>37 weeks) were therefore in the subgroup of babies delivered more than seven days after randomisation. Death and respiratory distress syndrome are rare in babies delivered at term; fewer outcomes would therefore occur in this subgroup, so a statistically significant difference would be less likely to be found. Hence, the lack of evidence of a difference in this group may simply reflect the much lower incidence of outcomes in babies born at term. The overall incidence of respiratory distress syndrome in the three subgroups in the most recent review8 is consistent with this argument—27.5% in the less than 24 hours subgroup, 16.4% for one to seven days, and 7.0% for more than seven days.
Subgroup comparisons did not use interaction tests
Subgroups with fewer trials or fewer events have greater uncertainty and are less likely to give statistically significant results than those with more trials or events, even if their treatment effects are exactly the same. Hence, using statistical significance to assess differences between subgroups is unreliable. Instead, statistical tests of interaction should always be used to assess subgroup differences, both in trials and systematic reviews.16
The time to delivery subgroups contained different sets of trials, which complicates the performance of interaction tests in these reviews. It would be expected that some women would deliver in each time period after randomisation in almost all trials, so that data should be available for each subgroup for each trial. However, in the Cochrane review, only five of 11 trials reported data for all subgroups.8 This could introduce bias. Firstly, one subgroup may, by chance, contain trials with larger treatment effects or more participants, making that subgroup more likely to show a significant treatment effect. This would give an impression of a difference between the subgroups, which would not be seen if data from all the trials were included in all subgroups. Secondly, a potential reporting bias exists—subgroup results may have been reported in the original trial papers because they were statistically significant, with non-significant subgroup results not being reported. Reviews would then tend to contain those subgroups that had significant results.
Subgroups were classified by an outcome variable
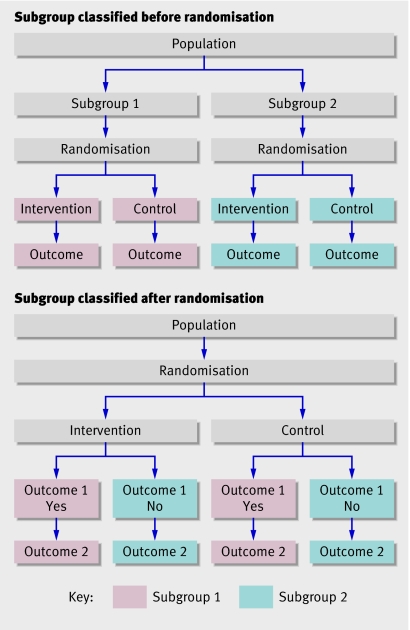
The time between randomisation and delivery is not known at trial entry, as it is not determined until birth. Subgroup analyses classified by variables that arise after randomisation are known to have a high risk of producing misleading results.17 18 Normally, subgroups are defined by variables known at randomisation (figure), and comparison between the arms of the trial within each subgroup is unbiased, because randomisation ensures the arms are balanced.
Different types of subgroup analysis. When subgroup analysis is classified by a variable known at randomisation (top), the composition of the intervention group and control group in each subgroup is determined by randomisation. When subgroup analysis is classified by a variable determined after randomisation (bottom), the composition of the two groups is determined by the presence or absence of outcome 1
However, if subgroups are defined by variables that arise after randomisation (outcome variables), the risk of bias is high (figure). Because membership of a subgroup depends on the presence or absence of an outcome, there is no way to ensure that the intervention and control groups within each subgroup are balanced, either in number of participants or baseline characteristics. Moreover, the subgroups may differ in important ways, as participants with or without a particular outcome are likely to be different. For example, women who deliver more than seven days after randomisation may differ from others in age, number of previous pregnancies, reasons for the risk of preterm birth, gestational age, or other factors. Any differences between subgroups in the effectiveness of the intervention may therefore be caused by differences in the composition of subgroups. None of the antenatal corticosteroid trials provided data comparing the baseline characteristics or other outcomes of the time to delivery subgroups, so the likelihood of bias cannot be assessed.
Whether the effects of antenatal corticosteroids change with time to delivery cannot be adequately investigated by the existing analyses, but a valid and straightforward method of analysis has been suggested.17 This uses standard techniques to determine which baseline characteristics, including randomised treatment, are related to the interval between randomisation and delivery, followed by standard subgroup analyses on any such variables identified. The original (individual patient) data from each trial would be needed; this would also allow analysis to be based on the exact time to birth rather than the arbitrary categories used so far. Reanalysis of individual patient data may help clarify whether the hypothesised association is real, and if so, suggest how long the effects of antenatal steroids persist.
The way forward
The widely accepted notion that the benefits of antenatal steroids decline with time to birth is based on analyses with serious methodological problems and may not be correct. Largely as a result of these analyses, many women and babies were given multiple courses of steroids during the 1990s, and we do not know whether this practice was beneficial or harmful. Results of the current trials of multiple versus single courses should answer this question, but the results of long term follow-up will not be available for several years. Until then, reanalysis of the data from the original trials may help to clarify whether effectiveness declines with time to delivery, and if so, over what timescale this occurs.
Two of the recently published trials of single versus multiple courses have contained subgroup analyses classified by outcome variables.12 19 These included analyses of subgroups based on gestational age at birth, and one trial claimed a significant benefit of multiple courses in babies born at less than 28 weeks. This may be misleading. Such analyses should be omitted from the reports of other trials and systematic reviews of multiple versus single courses or we risk repeating the errors made in the conclusions of subgroup analysis of the original antenatal steroid trials.
Summary points
Subgroup analyses in trials and systematic reviews of antenatal corticosteroids for neonatal lung disease suggested that effectiveness peaked one to seven days after treatment
This led to repeated treatment courses for women who did not deliver within a week
Methodological problems in these analyses mean that this conclusion may be wrong
Problems included not using interaction tests and classifying subgroups by variables arising after randomisation (outcome variables)
Awareness of such problems is low—two recent trials of repeated versus single courses of antenatal corticosteroids included analyses of subgroups classified by outcome variables
Thanks to Doug Altman and Finbar O'Callaghan for helpful comments on an earlier draft.
Contributions and sources: PB is a clinical trialist with a background in obstetrics and SG is a trialist and statistician. The authors worked together conducting clinical trials at the National Perinatal Epidemiology Unit (Oxford University) until 2005. This article originated in discussions about the evidence base underlying repeated courses of antenatal steroids with various colleagues while running a pilot study of this treatment. SG is guarantor.
Funding: The National Perinatal Epidemiology Unit is funded by the Department of Health in England. The views expressed in this paper are those of the authors and do not necessarily reflect the views of the Department of Health.
Competing interests: None declared.
Provenance and peer review: Non-commissioned; externally peer reviewed.
References
- 1.Crowley P. Promoting pulmonary maturity. In: Chalmers I, Enkin M, Keirse MJNC, eds. Effective care in pregnancy and childbirth Oxford: Oxford University Press, 1989:746-64.
- 2.Crowley P. The effects of corticosteroid administration before preterm delivery: an overview of the evidence from controlled trials. Br J Obstet Gynaecol 1990;97:11-25. [DOI] [PubMed] [Google Scholar]
- 3.Crowley PA. Antenatal corticosteroid therapy: a meta-analysis of the randomized trials, 1972 to 1994. Am J Obstet Gynecol 1995;173:322-35. [DOI] [PubMed] [Google Scholar]
- 4.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972;50:515-25. [PubMed] [Google Scholar]
- 5.Howie RN, Liggins GC. Clinical trial of antepartum betamethasone therapy for prevention of respiratory distress in pre-term infants. In: Anderson ABM, Beard RW, Brudenell JM, Dunn PM, eds. Pre-term labour London: RCOG, 1977:281-9.
- 6.Jobe AH. Glucocorticoids, inflammation and the perinatal lung. Semin Neonatol 2001;6:331-42. [DOI] [PubMed] [Google Scholar]
- 7.Grier DG, Halliday HL. Effects of glucocorticoids on fetal and neonatal lung development. Treat Respir Med 2004;3:295-306. [DOI] [PubMed] [Google Scholar]
- 8.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev 2006;(3):CD004454. [DOI] [PubMed]
- 9.Brocklehurst P, Gates S, McKenzie-McHarg K, Alfirevic Z, Chamberlain G. Are we using multiple courses of antenatal corticosteroids? A survey of practice in the UK. Br J Obstet Gynaecol 1999;106:977-9. [DOI] [PubMed] [Google Scholar]
- 10.Quinlivan JA, Evans SF, Dunlop SA, Beazley LD, Newnham JP. Use of corticosteroids by Australian obstetricians—a survey of clinical practice. Aust N Z J Obstet Gynaecol 1998;38:1-7. [DOI] [PubMed] [Google Scholar]
- 11.Crowther CA, Harding JE. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for preventing neonatal respiratory disease. Cochrane Database Syst Rev 2000;(2):CD003935. [DOI] [PubMed]
- 12.Wapner RJ, Sorokin Y, Thom EA, Johnson F, Dudley DJ, Spong CY, et al. Single versus weekly courses of antenatal corticosteroids: evaluation of safety and efficacy. Am J Obstet Gynecol 2006;195:633-42. [DOI] [PubMed] [Google Scholar]
- 13.Crowther CA, Haslam RR, Hiller JE, Doyle LW, Robinson JS; Australasian Collaborative Trial of Repeat Doses of Steroids (ACTORDS) Study Group. Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: a randomised controlled trial. Lancet 2006;367:1913-9. [DOI] [PubMed] [Google Scholar]
- 14.Monton S, Arukumaran S. Neonatal respiratory distress syndrome. Lancet 2006;367:1878-9. [DOI] [PubMed] [Google Scholar]
- 15.NIH Consensus Development Panel. Antenatal corticosteroids revisited: repeat courses—National Institutes of Health consensus development conference statement, August 17-18, 2000. Obstet Gynecol 2001;98:144-50. [DOI] [PubMed] [Google Scholar]
- 16.Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, eds. Systematic reviews in health care: meta-analysis in context London: BMJ Publishing Group, 2001
- 17.Rochon J. Issues in adjusting for covariates arising postrandomisation in clinical trials. Drug Inform J 1999;33:1219-28. [Google Scholar]
- 18.Van Walraven C, Davis D, Forster AJ, Wells GA. Time-dependent bias was common in survival analyses published in leading clinical journals. J Clin Epidemiol 2004;57:672-82. [DOI] [PubMed] [Google Scholar]
- 19.Guinn DA, Atkinson MW, Sullivan L, Lee M, Macgregor S, Parilla BV, et al. Single vs weekly courses of antenatal corticosteroids for women at risk of preterm delivery: a randomized controlled trial. JAMA 2001;286:1581-7. [DOI] [PubMed] [Google Scholar]