Abstract
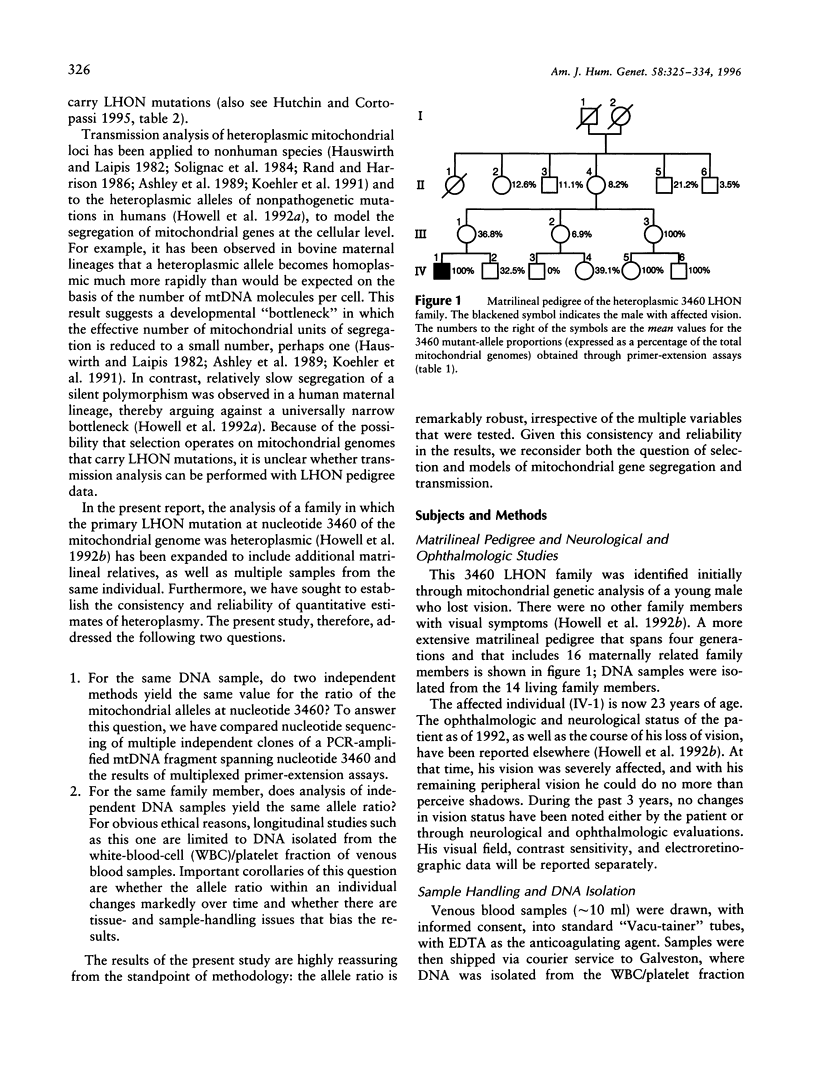
Nucleotide-sequencing and multiplexed primer-extension assays have been used to quantitate the mutant-allele frequency in 14 maternal relatives, spanning three generations, from a family that is heteroplasmic for the primary Leber hereditary optic neuropathy (LHON) mutation at nucleotide 3460 of the mitochondrial genome. There was excellent agreement between the values that were obtained with the two different methods. The longitudinal study shows that the mutant-allele frequency was constant within individual family members over a sampling period of 3.5 years. Second, although there was an overall increase in the mutant-allele frequency in successive generations, segregation in the direction of the mutant allele was not invariant, and there was one instance in which there was a significant decrease in the frequency from parent to offspring. From these two sets of results, and from previous studies of heteroplasmic LHON families, we conclude that there is no evidence for a marked selective pressure that determines the replication, segregation, or transmission of primary LHON mutations to white blood cells and platelets. Instead, the mtDNA molecules are most likely to replicate and segregate under conditions of random drift at the cellular level. Finally, the pattern of transmission in this maternal lineage is compatible with a developmental bottleneck model in which the number of mitochondrial units of segregation in the female germ line is relatively small in relation to the number of mtDNA molecules within a cell. However, this is not an invariant pattern for humans, and simple models of mitochondrial gene transmission are inappropriate at the present time.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Ashley M. V., Laipis P. J., Hauswirth W. W. Rapid segregation of heteroplasmic bovine mitochondria. Nucleic Acids Res. 1989 Sep 25;17(18):7325–7331. doi: 10.1093/nar/17.18.7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauswirth W. W., Laipis P. J. Mitochondrial DNA polymorphism in a maternal lineage of Holstein cows. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4686–4690. doi: 10.1073/pnas.79.15.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi Y., Linn S. Purification of all forms of HeLa cell mitochondrial DNA and assessment of damage to it caused by hydrogen peroxide treatment of mitochondria or cells. J Biol Chem. 1995 Apr 7;270(14):7950–7956. doi: 10.1074/jbc.270.14.7950. [DOI] [PubMed] [Google Scholar]
- Howell N., Bindoff L. A., McCullough D. A., Kubacka I., Poulton J., Mackey D., Taylor L., Turnbull D. M. Leber hereditary optic neuropathy: identification of the same mitochondrial ND1 mutation in six pedigrees. Am J Hum Genet. 1991 Nov;49(5):939–950. [PMC free article] [PubMed] [Google Scholar]
- Howell N., Halvorson S., Kubacka I., McCullough D. A., Bindoff L. A., Turnbull D. M. Mitochondrial gene segregation in mammals: is the bottleneck always narrow? Hum Genet. 1992 Sep-Oct;90(1-2):117–120. doi: 10.1007/BF00210753. [DOI] [PubMed] [Google Scholar]
- Howell N., McCullough D., Bodis-Wollner I. Molecular genetic analysis of a sporadic case of Leber hereditary optic neuropathy. Am J Hum Genet. 1992 Feb;50(2):443–446. [PMC free article] [PubMed] [Google Scholar]
- Howell N., Xu M., Halvorson S., Bodis-Wollner I., Sherman J. A heteroplasmic LHON family: tissue distribution and transmission of the 11778 mutation. Am J Hum Genet. 1994 Jul;55(1):203–206. [PMC free article] [PubMed] [Google Scholar]
- Huoponen K., Juvonen V., Iitiä A., Dahlen P., Siitari H., Aula P., Nikoskelainen E., Savontaus M. L. Time-resolved fluorometry in the diagnosis of Leber hereditary optic neuroretinopathy. Hum Mutat. 1994;3(1):29–36. doi: 10.1002/humu.1380030106. [DOI] [PubMed] [Google Scholar]
- Hutchin T., Cortopassi G. A mitochondrial DNA clone is associated with increased risk for Alzheimer disease. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):6892–6895. doi: 10.1073/pnas.92.15.6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvonen V., Huoponen K., Syvänen A. C., Nikoskelainen E., Savontaus M. L. Quantification of point mutations associated with Leber hereditary optic neuroretinopathy by solid-phase minisequencing. Hum Genet. 1994 Jan;93(1):16–20. doi: 10.1007/BF00218906. [DOI] [PubMed] [Google Scholar]
- Koehler C. M., Lindberg G. L., Brown D. R., Beitz D. C., Freeman A. E., Mayfield J. E., Myers A. M. Replacement of bovine mitochondrial DNA by a sequence variant within one generation. Genetics. 1991 Sep;129(1):247–255. doi: 10.1093/genetics/129.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott M. T., Voljavec A. S., Wallace D. C. Variable genotype of Leber's hereditary optic neuropathy patients. Am J Ophthalmol. 1990 Jun 15;109(6):625–631. doi: 10.1016/s0002-9394(14)72429-8. [DOI] [PubMed] [Google Scholar]
- Mackey D. A., Buttery R. G. Leber hereditary optic neuropathy in Australia. Aust N Z J Ophthalmol. 1992 Aug;20(3):177–184. doi: 10.1111/j.1442-9071.1992.tb00937.x. [DOI] [PubMed] [Google Scholar]
- Mashima Y., Saga M., Hiida Y., Oguchi Y., Wakakura M., Kudoh J., Shimizu N. Quantitative determination of heteroplasmy in Leber's hereditary optic neuropathy by single-strand conformation polymorphism. Invest Ophthalmol Vis Sci. 1995 Jul;36(8):1714–1720. [PubMed] [Google Scholar]
- Matthews P. M., Brown R. M., Morten K., Marchington D., Poulton J., Brown G. Intracellular heteroplasmy for disease-associated point mutations in mtDNA: implications for disease expression and evidence for mitotic segregation of heteroplasmic units of mtDNA. Hum Genet. 1995 Sep;96(3):261–268. doi: 10.1007/BF00210404. [DOI] [PubMed] [Google Scholar]
- Nass M. M. Mitochondrial DNA. I. Intramitochondrial distribution and structural relations of single- and double-length circular DNA. J Mol Biol. 1969 Jun 28;42(3):521–528. doi: 10.1016/0022-2836(69)90240-x. [DOI] [PubMed] [Google Scholar]
- Newman N. J. Leber's hereditary optic neuropathy. New genetic considerations. Arch Neurol. 1993 May;50(5):540–548. doi: 10.1001/archneur.1993.00540050082021. [DOI] [PubMed] [Google Scholar]
- Poulton J., Morten K. Noninvasive diagnosis of the MELAS syndrome from blood DNA. Ann Neurol. 1993 Jul;34(1):116–116. doi: 10.1002/ana.410340124. [DOI] [PubMed] [Google Scholar]
- Rand D. M., Harrison R. G. Mitochondrial DNA transmission genetics in crickets. Genetics. 1986 Nov;114(3):955–970. doi: 10.1093/genetics/114.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan-Eva P., Sanders M. D., Govan G. G., Sweeney M. G., Da Costa J., Harding A. E. The clinical features of Leber's hereditary optic neuropathy defined by the presence of a pathogenic mitochondrial DNA mutation. Brain. 1995 Apr;118(Pt 2):319–337. doi: 10.1093/brain/118.2.319. [DOI] [PubMed] [Google Scholar]
- Satoh M., Kuroiwa T. Organization of multiple nucleoids and DNA molecules in mitochondria of a human cell. Exp Cell Res. 1991 Sep;196(1):137–140. doi: 10.1016/0014-4827(91)90467-9. [DOI] [PubMed] [Google Scholar]
- Smith K. H., Johns D. R., Heher K. L., Miller N. R. Heteroplasmy in Leber's hereditary optic neuropathy. Arch Ophthalmol. 1993 Nov;111(11):1486–1490. doi: 10.1001/archopht.1993.01090110052022. [DOI] [PubMed] [Google Scholar]
- Torroni A., Wallace D. C. Mitochondrial DNA variation in human populations and implications for detection of mitochondrial DNA mutations of pathological significance. J Bioenerg Biomembr. 1994 Jun;26(3):261–271. doi: 10.1007/BF00763098. [DOI] [PubMed] [Google Scholar]
- Vilkki J., Savontaus M. L., Nikoskelainen E. K. Segregation of mitochondrial genomes in a heteroplasmic lineage with Leber hereditary optic neuroretinopathy. Am J Hum Genet. 1990 Jul;47(1):95–100. [PMC free article] [PubMed] [Google Scholar]
- Wallace D. C., Singh G., Lott M. T., Hodge J. A., Schurr T. G., Lezza A. M., Elsas L. J., 2nd, Nikoskelainen E. K. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988 Dec 9;242(4884):1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- Zhu D. P., Economou E. P., Antonarakis S. E., Maumenee I. H. Mitochondrial DNA mutation and heteroplasmy in type I Leber hereditary optic neuropathy. Am J Med Genet. 1992 Jan 15;42(2):173–179. doi: 10.1002/ajmg.1320420208. [DOI] [PubMed] [Google Scholar]



