Abstract
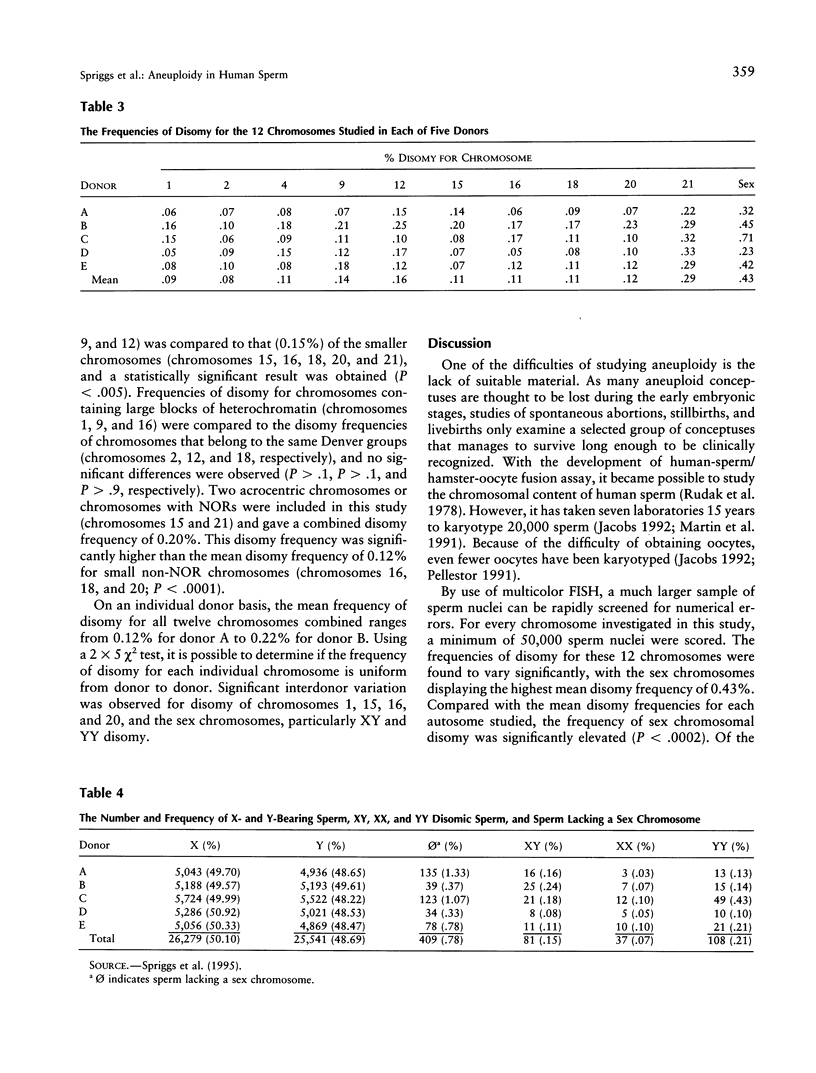
While it is known that all chromosomes are susceptible to meiotic nondisjunction, it is not clear whether all chromosomes display the same frequency of nondisjunction. By use of multicolor FISH and chromosome-specific probes, the frequency of disomy in human sperm was determined for chromosomes 1, 2, 4, 9, 12, 15, 16, 18, 20, and 21, and the sex chromosomes. A minimum of 10,000 sperm nuclei were scored from each of five healthy, chromosomally normal donors for every chromosome studied, giving a total of 418,931 sperm nuclei. The mean frequencies of disomy obtained were 0.09% for chromosome 1; 0.08% for chromosome 2; 0.11% for chromosome 4; 0.14% for chromosome 9; 0.16% for chromosome 12; 0.11% for chromosomes 15, 16, and 18; 0.12% for chromosome 20; 0.29% for chromosome 21; and 0.43% for the sex chromosomes. Data for chromosomes 1, 12, 15, and 18, and the sex chromosomes have been published elsewhere. When the mean frequencies of disomy were compared, the sex chromosomes and chromosome 21 had significantly higher frequencies of disomy than that of any other autosome studied. These results corroborate the pooled data obtained from human sperm karyotypes and suggest that the sex chromosome bivalent and the chromosome 21 bivalent are more susceptible to nondisjunction during spermatogenesis. From these findings, theories proposed to explain the variable incidence of nondisjunction can be supported or discarded as improbable.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonarakis S. E., Kittur S. D., Metaxotou C., Watkins P. C., Patel A. S. Analysis of DNA haplotypes suggests a genetic predisposition to trisomy 21 associated with DNA sequences on chromosome 21. Proc Natl Acad Sci U S A. 1985 May;82(10):3360–3364. doi: 10.1073/pnas.82.10.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonarakis S. E. Parental origin of the extra chromosome in trisomy 21 as indicated by analysis of DNA polymorphisms. Down Syndrome Collaborative Group. N Engl J Med. 1991 Mar 28;324(13):872–876. doi: 10.1056/NEJM199103283241302. [DOI] [PubMed] [Google Scholar]
- Baldini A., Rocchi M., Archidiacono N., Miller O. J., Miller D. A. A human alpha satellite DNA subset specific for chromosome 12. Am J Hum Genet. 1990 Apr;46(4):784–788. [PMC free article] [PubMed] [Google Scholar]
- Choo K. H., Earle E., Vissel B., Filby R. G. Identification of two distinct subfamilies of alpha satellite DNA that are highly specific for human chromosome 15. Genomics. 1990 Jun;7(2):143–151. doi: 10.1016/0888-7543(90)90534-2. [DOI] [PubMed] [Google Scholar]
- Cooke H. J., Hindley J. Cloning of human satellite III DNA: different components are on different chromosomes. Nucleic Acids Res. 1979 Jul 25;6(10):3177–3197. doi: 10.1093/nar/6.10.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J. M., Harvey J. F., Lindenbaum R. H., Boyd P. A., Jacobs P. A. Molecular studies of trisomy 18. Am J Hum Genet. 1993 Jun;52(6):1139–1144. [PMC free article] [PubMed] [Google Scholar]
- Ford J. H., Lester P. Factors affecting the displacement of human chromosomes from the metaphase plate. Cytogenet Cell Genet. 1982;33(4):327–332. doi: 10.1159/000131779. [DOI] [PubMed] [Google Scholar]
- García M., Dietrich A., Pujol R., Egozcue J. Nucleolar structures in chromosome and SC preparations from human oocytes at first meiotic prophase. Hum Genet. 1989 May;82(2):147–153. doi: 10.1007/BF00284048. [DOI] [PubMed] [Google Scholar]
- Greig G. M., England S. B., Bedford H. M., Willard H. F. Chromosome-specific alpha satellite DNA from the centromere of human chromosome 16. Am J Hum Genet. 1989 Dec;45(6):862–872. [PMC free article] [PubMed] [Google Scholar]
- Hassold T. J., Jacobs P. A. Trisomy in man. Annu Rev Genet. 1984;18:69–97. doi: 10.1146/annurev.ge.18.120184.000441. [DOI] [PubMed] [Google Scholar]
- Hassold T. J., Pettay D., Freeman S. B., Grantham M., Takaesu N. Molecular studies of non-disjunction in trisomy 16. J Med Genet. 1991 Mar;28(3):159–162. doi: 10.1136/jmg.28.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassold T. J., Sherman S. L., Pettay D., Page D. C., Jacobs P. A. XY chromosome nondisjunction in man is associated with diminished recombination in the pseudoautosomal region. Am J Hum Genet. 1991 Aug;49(2):253–260. [PMC free article] [PubMed] [Google Scholar]
- Hassold T., Pettay D., Robinson A., Uchida I. Molecular studies of parental origin and mosaicism in 45,X conceptuses. Hum Genet. 1992 Aug;89(6):647–652. doi: 10.1007/BF00221956. [DOI] [PubMed] [Google Scholar]
- Jabs E. W., Goble C. A., Cutting G. R. Macromolecular organization of human centromeric regions reveals high-frequency, polymorphic macro DNA repeats. Proc Natl Acad Sci U S A. 1989 Jan;86(1):202–206. doi: 10.1073/pnas.86.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs P. A., Betts P. R., Cockwell A. E., Crolla J. A., Mackenzie M. J., Robinson D. O., Youings S. A. A cytogenetic and molecular reappraisal of a series of patients with Turner's syndrome. Ann Hum Genet. 1990 Jul;54(Pt 3):209–223. doi: 10.1111/j.1469-1809.1990.tb00379.x. [DOI] [PubMed] [Google Scholar]
- Laurie D. A., Hultén M. A. Further studies on chiasma distribution and interference in the human male. Ann Hum Genet. 1985 Jul;49(Pt 3):203–214. doi: 10.1111/j.1469-1809.1985.tb01694.x. [DOI] [PubMed] [Google Scholar]
- Lorda-Sanchez I., Binkert F., Maechler M., Robinson W. P., Schinzel A. A. Reduced recombination and paternal age effect in Klinefelter syndrome. Hum Genet. 1992 Jul;89(5):524–530. doi: 10.1007/BF00219178. [DOI] [PubMed] [Google Scholar]
- MacDonald M., Hassold T., Harvey J., Wang L. H., Morton N. E., Jacobs P. The origin of 47,XXY and 47,XXX aneuploidy: heterogeneous mechanisms and role of aberrant recombination. Hum Mol Genet. 1994 Aug;3(8):1365–1371. doi: 10.1093/hmg/3.8.1365. [DOI] [PubMed] [Google Scholar]
- Martin R. H., Ko E., Rademaker A. Distribution of aneuploidy in human gametes: comparison between human sperm and oocytes. Am J Med Genet. 1991 Jun 1;39(3):321–331. doi: 10.1002/ajmg.1320390315. [DOI] [PubMed] [Google Scholar]
- Mirre C., Hartung M., Stahl A. Association of ribosomal genes in the fibrillar center of the nucleolus: a factor influencing translocation and nondisjunction in the human meiotic oocyte. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6017–6021. doi: 10.1073/pnas.77.10.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nöthen M. M., Eggermann T., Erdmann J., Eiben B., Hofmann D., Propping P., Schwanitz G. Retrospective study of the parental origin of the extra chromosome in trisomy 18 (Edwards syndrome). Hum Genet. 1993 Oct;92(4):347–349. doi: 10.1007/BF01247332. [DOI] [PubMed] [Google Scholar]
- POLANI P. E., BRIGGS J. H., FORD C. E., CLARKE C. M., BERG J. M. A Mongol girl with 46 chromosomes. Lancet. 1960 Apr 2;1(7127):721–724. doi: 10.1016/s0140-6736(60)90614-0. [DOI] [PubMed] [Google Scholar]
- Pellestor F. Frequency and distribution of aneuploidy in human female gametes. Hum Genet. 1991 Jan;86(3):283–288. doi: 10.1007/BF00202410. [DOI] [PubMed] [Google Scholar]
- Rudak E., Jacobs P. A., Yanagimachi R. Direct analysis of the chromosome constitution of human spermatozoa. Nature. 1978 Aug 31;274(5674):911–913. doi: 10.1038/274911a0. [DOI] [PubMed] [Google Scholar]
- Sacchi N., Gusella J. F., Perroni L., Bricarelli F. D., Papas T. S. Lack of evidence for association of meiotic nondisjunction with particular DNA haplotypes on chromosome 21. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4794–4798. doi: 10.1073/pnas.85.13.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmickel R. D., Gonzalez I. L., Erickson J. M. Nucleolus organizing genes on chromosome 21: recombination and nondisjunction. Ann N Y Acad Sci. 1985;450:121–131. doi: 10.1111/j.1749-6632.1985.tb21488.x. [DOI] [PubMed] [Google Scholar]
- Sherman S. L., Takaesu N., Freeman S. B., Grantham M., Phillips C., Blackston R. D., Jacobs P. A., Cockwell A. E., Freeman V., Uchida I. Trisomy 21: association between reduced recombination and nondisjunction. Am J Hum Genet. 1991 Sep;49(3):608–620. [PMC free article] [PubMed] [Google Scholar]
- Spriggs E. L., Rademaker A. W., Martin R. H. Aneuploidy in human sperm: results of two-and three-color fluorescence in situ hybridization using centromeric probes for chromosomes 1, 12, 15, 18, X, and Y. Cytogenet Cell Genet. 1995;71(1):47–53. doi: 10.1159/000134060. [DOI] [PubMed] [Google Scholar]
- Tagarro I., Fernández-Peralta A. M., González-Aguilera J. J. Chromosomal localization of human satellites 2 and 3 by a FISH method using oligonucleotides as probes. Hum Genet. 1994 Apr;93(4):383–388. doi: 10.1007/BF00201662. [DOI] [PubMed] [Google Scholar]
- Watt J. L., Templeton A. A., Messinis I., Bell L., Cunningham P., Duncan R. O. Trisomy 1 in an eight cell human pre-embryo. J Med Genet. 1987 Jan;24(1):60–64. doi: 10.1136/jmg.24.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza M. V., Jacobs P. A., James R. S., Rogan P., Sherman S., Hassold T. Nondisjunction of human acrocentric chromosomes: studies of 432 trisomic fetuses and liveborns. Hum Genet. 1994 Oct;94(4):411–417. doi: 10.1007/BF00201603. [DOI] [PubMed] [Google Scholar]



