Abstract
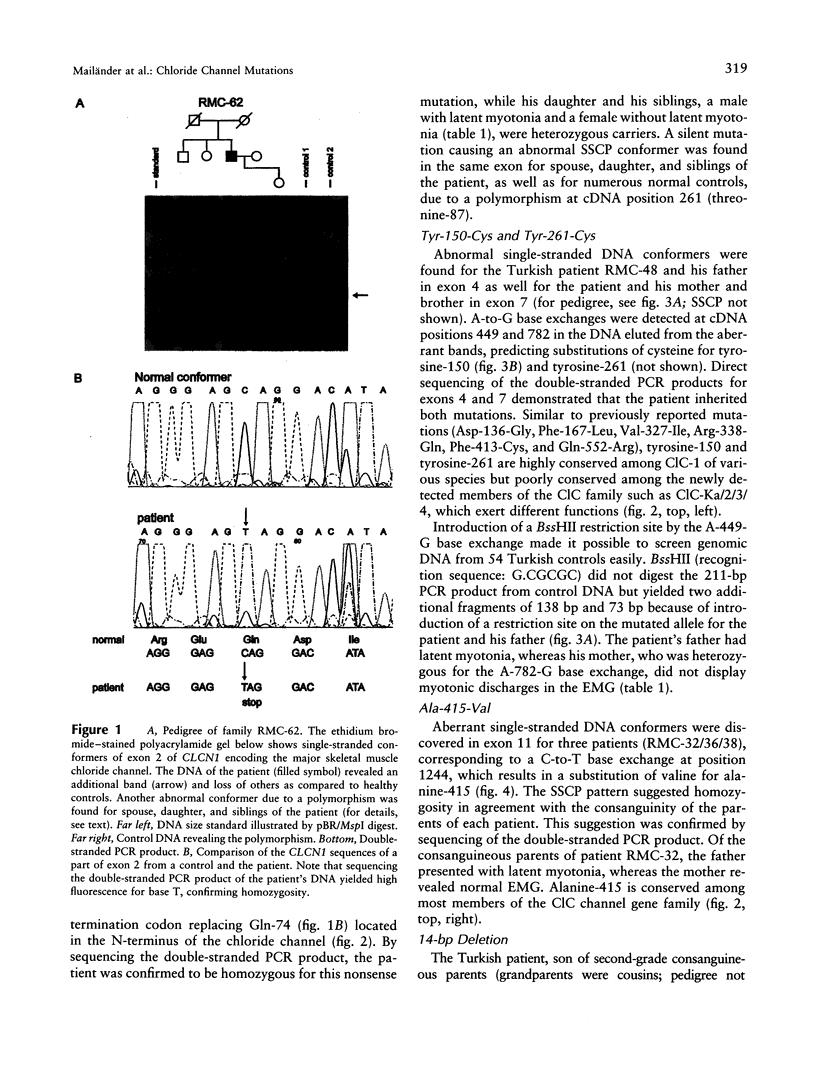
Mutations within CLCN1, the gene encoding the major skeletal muscle chloride channel, cause either dominant Thomsen disease or recessive Becker-type myotonia, which are sometimes difficult to discriminate, because of reduced penetrance or lower clinical expressivity in females. We screened DNA of six unrelated Becker patients and found four novel CLCN1 mutations (Gln-74-Stop, Tyr-150-Cys, Tyr-261-Cys, and Ala-415-Val) and a previously reported 14-bp deletion. Five patients were homozygous for the changes (Gln-74-Stop, Ala-415-Val, and 14-bp deletion), four of them due to parental consanguinity. The sixth patient revealed compound heterozygosity for Tyr-150-Cys and Tyr-261-Cys. Heterozygous carriers of the Becker mutations did not display any clinical symptoms of myotonia. However, all heterozygous males, but none of the heterozygous females, exhibited myotonic discharges in the electromyogram suggesting (i) a gene dosage effect of the mutations on the chloride conductance and (ii) male predominance of subclinical myotonia. Furthermore, we report a novel Gly-200-Arg mutation resulting in a dominant phenotype in a male and a partially dominant phenotype in his mother. We discuss potential causes of the gender preference and the molecular mechanisms that may determine the mode of inheritance.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKER P. E. Zur Frage der Heterogenie der erblichen Myotonien. Nervenarzt. 1957 Oct 20;28(10):455–460. [PubMed] [Google Scholar]
- Bryant S. H. Cable properties of external intercostal muscle fibres from myotonic and nonmyotonic goats. J Physiol. 1969 Oct;204(3):539–550. doi: 10.1113/jphysiol.1969.sp008930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disteche C. M. Escape from X inactivation in human and mouse. Trends Genet. 1995 Jan;11(1):17–22. doi: 10.1016/s0168-9525(00)88981-7. [DOI] [PubMed] [Google Scholar]
- George A. L., Jr, Crackower M. A., Abdalla J. A., Hudson A. J., Ebers G. C. Molecular basis of Thomsen's disease (autosomal dominant myotonia congenita). Nat Genet. 1993 Apr;3(4):305–310. doi: 10.1038/ng0493-305. [DOI] [PubMed] [Google Scholar]
- George A. L., Jr, Sloan-Brown K., Fenichel G. M., Mitchell G. A., Spiegel R., Pascuzzi R. M. Nonsense and missense mutations of the muscle chloride channel gene in patients with myotonia congenita. Hum Mol Genet. 1994 Nov;3(11):2071–2072. [PubMed] [Google Scholar]
- Gronemeier M., Condie A., Prosser J., Steinmeyer K., Jentsch T. J., Jockusch H. Nonsense and missense mutations in the muscular chloride channel gene Clc-1 of myotonic mice. J Biol Chem. 1994 Feb 25;269(8):5963–5967. [PubMed] [Google Scholar]
- Heine R., George A. L., Jr, Pika U., Deymeer F., Rüdel R., Lehmann-Horn F. Proof of a non-functional muscle chloride channel in recessive myotonia congenita (Becker) by detection of a 4 base pair deletion. Hum Mol Genet. 1994 Jul;3(7):1123–1128. doi: 10.1093/hmg/3.7.1123. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Lehmann-Horn F., Rüdel R. Overexcited or inactive: ion channels in muscle disease. Cell. 1995 Mar 10;80(5):681–686. doi: 10.1016/0092-8674(95)90345-3. [DOI] [PubMed] [Google Scholar]
- Kawasaki M., Uchida S., Monkawa T., Miyawaki A., Mikoshiba K., Marumo F., Sasaki S. Cloning and expression of a protein kinase C-regulated chloride channel abundantly expressed in rat brain neuronal cells. Neuron. 1994 Mar;12(3):597–604. doi: 10.1016/0896-6273(94)90215-1. [DOI] [PubMed] [Google Scholar]
- Kieferle S., Fong P., Bens M., Vandewalle A., Jentsch T. J. Two highly homologous members of the ClC chloride channel family in both rat and human kidney. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):6943–6947. doi: 10.1073/pnas.91.15.6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M. C., Steinmeyer K., Lorenz C., Ricker K., Wolf F., Otto M., Zoll B., Lehmann-Horn F., Grzeschik K. H., Jentsch T. J. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science. 1992 Aug 7;257(5071):797–800. doi: 10.1126/science.1379744. [DOI] [PubMed] [Google Scholar]
- Kwieciński H., Lehmann-Horn F., Rüdel R. Drug-induced myotonia in human intercostal muscle. Muscle Nerve. 1988 Jun;11(6):576–581. doi: 10.1002/mus.880110609. [DOI] [PubMed] [Google Scholar]
- Lehmann-Horn F., Mailänder V., Heine R., George A. L. Myotonia levior is a chloride channel disorder. Hum Mol Genet. 1995 Aug;4(8):1397–1402. doi: 10.1093/hmg/4.8.1397. [DOI] [PubMed] [Google Scholar]
- Lipicky R. J., Bryant S. H., Salmon J. H. Cable parameters, sodium, potassium, chloride, and water content, and potassium efflux in isolated external intercostal muscle of normal volunteers and patients with myotonia congenita. J Clin Invest. 1971 Oct;50(10):2091–2103. doi: 10.1172/JCI106703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz C., Meyer-Kleine C., Steinmeyer K., Koch M. C., Jentsch T. J. Genomic organization of the human muscle chloride channel CIC-1 and analysis of novel mutations leading to Becker-type myotonia. Hum Mol Genet. 1994 Jun;3(6):941–946. doi: 10.1093/hmg/3.6.941. [DOI] [PubMed] [Google Scholar]
- Mehrke G., Brinkmeier H., Jockusch H. The myotonic mouse mutant ADR: electrophysiology of the muscle fiber. Muscle Nerve. 1988 May;11(5):440–446. doi: 10.1002/mus.880110505. [DOI] [PubMed] [Google Scholar]
- Meyer-Kleine C., Ricker K., Otto M., Koch M. C. A recurrent 14 bp deletion in the CLCN1 gene associated with generalized myotonia (Becker). Hum Mol Genet. 1994 Jun;3(6):1015–1016. doi: 10.1093/hmg/3.6.1015. [DOI] [PubMed] [Google Scholar]
- Middleton R. E., Pheasant D. J., Miller C. Purification, reconstitution, and subunit composition of a voltage-gated chloride channel from Torpedo electroplax. Biochemistry. 1994 Nov 15;33(45):13189–13198. doi: 10.1021/bi00249a005. [DOI] [PubMed] [Google Scholar]
- Palade P. T., Barchi R. L. On the inhibition of muscle membrane chloride conductance by aromatic carboxylic acids. J Gen Physiol. 1977 Jun;69(6):879–896. doi: 10.1085/jgp.69.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüdel R., Ricker K., Lehmann-Horn F. Transient weakness and altered membrane characteristic in recessive generalized myotonia (Becker). Muscle Nerve. 1988 Mar;11(3):202–211. doi: 10.1002/mus.880110303. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K., Klocke R., Ortland C., Gronemeier M., Jockusch H., Gründer S., Jentsch T. J. Inactivation of muscle chloride channel by transposon insertion in myotonic mice. Nature. 1991 Nov 28;354(6351):304–308. doi: 10.1038/354304a0. [DOI] [PubMed] [Google Scholar]
- Steinmeyer K., Lorenz C., Pusch M., Koch M. C., Jentsch T. J. Multimeric structure of ClC-1 chloride channel revealed by mutations in dominant myotonia congenita (Thomsen). EMBO J. 1994 Feb 15;13(4):737–743. doi: 10.1002/j.1460-2075.1994.tb06315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streib E. W., Sun S. F. EMG in detection of heterozygote carriers of recessive generalized myotonia. Muscle Nerve. 1982 Feb;5(2):179–181. [PubMed] [Google Scholar]
- Thiemann A., Gründer S., Pusch M., Jentsch T. J. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature. 1992 Mar 5;356(6364):57–60. doi: 10.1038/356057a0. [DOI] [PubMed] [Google Scholar]
- Yap E. P., McGee J. O. Nonisotopic SSCP detection in PCR products by ethidium bromide staining. Trends Genet. 1992 Feb;8(2):49–49. doi: 10.1016/0168-9525(92)90339-6. [DOI] [PubMed] [Google Scholar]
- Zellweger H., Pavone L., Biondi A., Cimino V., Gullotta F., Hart M., Ionasescu V., Mollica F., Schieken R. Autosomal recessive generalized myotonia. Muscle Nerve. 1980 Mar-Apr;3(2):176–180. doi: 10.1002/mus.880030212. [DOI] [PubMed] [Google Scholar]
- van Slegtenhorst M. A., Bassi M. T., Borsani G., Wapenaar M. C., Ferrero G. B., de Conciliis L., Rugarli E. I., Grillo A., Franco B., Zoghbi H. Y. A gene from the Xp22.3 region shares homology with voltage-gated chloride channels. Hum Mol Genet. 1994 Apr;3(4):547–552. doi: 10.1093/hmg/3.4.547. [DOI] [PubMed] [Google Scholar]