Abstract
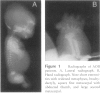
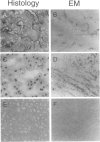
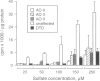
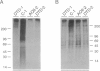
Atelosteogenesis type II (AO II) is a neonatally lethal chondrodysplasia whose clinical and histological characteristics resemble those of another chondrodysplasia, the much less severe diastrophic dysplasia (DTD). The similarity suggests a shared pathogenesis involving lesions in the same biochemical pathway and perhaps the same gene. DTD is caused by mutations in the recently identified diastrophic dysplasia sulfate-transporter gene (DTDST). Here, we report that AOII patients also have DTDST mutations, which lead to defective uptake of inorganic sulfate and insufficient sulfation of macromolecules by patient mesenchymal cells in vitro. Together with our recent observation that a third even more severe chondrodysplasia, achondrogenesis type IB, is also caused by mutations in DTDST, these results demonstrate a phenotypic series of three chondrodysplasias of increasing severity caused by lesions in a single sulfate-transporter gene. The severity of the phenotype appears to be correlated with the predicted effect of the mutations on the residual activity of the DTDST protein.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borochowitz Z., Lachman R., Adomian G. E., Spear G., Jones K., Rimoin D. L. Achondrogenesis type I: delineation of further heterogeneity and identification of two distinct subgroups. J Pediatr. 1988 Jan;112(1):23–31. doi: 10.1016/s0022-3476(88)80113-6. [DOI] [PubMed] [Google Scholar]
- Cole D. E., Scriver C. R. Microassay of inorganic sulfate in biological fluids by controlled flow anion chromatography. J Chromatogr. 1981 Oct 9;225(2):359–367. doi: 10.1016/s0378-4347(00)80284-4. [DOI] [PubMed] [Google Scholar]
- Dietrich W., Katz H., Lincoln S. E., Shin H. S., Friedman J., Dracopoli N. C., Lander E. S. A genetic map of the mouse suitable for typing intraspecific crosses. Genetics. 1992 Jun;131(2):423–447. doi: 10.1093/genetics/131.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgavish A., Smith J. B., Pillion D. J., Meezan E. Sulfate transport in human lung fibroblasts (IMR-90). J Cell Physiol. 1985 Nov;125(2):243–250. doi: 10.1002/jcp.1041250211. [DOI] [PubMed] [Google Scholar]
- Erlebacher A., Filvaroff E. H., Gitelman S. E., Derynck R. Toward a molecular understanding of skeletal development. Cell. 1995 Feb 10;80(3):371–378. doi: 10.1016/0092-8674(95)90487-5. [DOI] [PubMed] [Google Scholar]
- Esko J. D., Elgavish A., Prasthofer T., Taylor W. H., Weinke J. L. Sulfate transport-deficient mutants of Chinese hamster ovary cells. Sulfation of glycosaminoglycans dependent on cysteine. J Biol Chem. 1986 Nov 25;261(33):15725–15733. [PubMed] [Google Scholar]
- Gray M. L., Pizzanelli A. M., Grodzinsky A. J., Lee R. C. Mechanical and physiochemical determinants of the chondrocyte biosynthetic response. J Orthop Res. 1988;6(6):777–792. doi: 10.1002/jor.1100060602. [DOI] [PubMed] [Google Scholar]
- Horton W. A., Rimoin D. L., Lachman R. S., Skovby F., Hollister D. W., Spranger J., Scott C. I., Hall J. G. The phenotypic variability of diastrophic dysplasia. J Pediatr. 1978 Oct;93(4):609–613. doi: 10.1016/s0022-3476(78)80896-8. [DOI] [PubMed] [Google Scholar]
- Humphries D. E., Silbert C. K., Silbert J. E. Sulphation by cultured cells. Cysteine, cysteinesulphinic acid and sulphite as sources for proteoglycan sulphate. Biochem J. 1988 May 15;252(1):305–308. doi: 10.1042/bj2520305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hästbacka J., de la Chapelle A., Mahtani M. M., Clines G., Reeve-Daly M. P., Daly M., Hamilton B. A., Kusumi K., Trivedi B., Weaver A. The diastrophic dysplasia gene encodes a novel sulfate transporter: positional cloning by fine-structure linkage disequilibrium mapping. Cell. 1994 Sep 23;78(6):1073–1087. doi: 10.1016/0092-8674(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Mortier G. R., Wilkin D. J., Wilcox W. R., Rimoin D. L., Lachman R. S., Eyre D. R., Cohn D. H. A radiographic, morphologic, biochemical and molecular analysis of a case of achondrogenesis type II resulting from substitution for a glycine residue (Gly691-->Arg) in the type II collagen trimer. Hum Mol Genet. 1995 Feb;4(2):285–288. doi: 10.1093/hmg/4.2.285. [DOI] [PubMed] [Google Scholar]
- Nores J. A., Rotmensch S., Romero R., Avila C., Inati M., Hobbins J. C. Atelosteogenesis type II: sonographic and radiological correlation. Prenat Diagn. 1992 Sep;12(9):741–753. doi: 10.1002/pd.1970120907. [DOI] [PubMed] [Google Scholar]
- Pillion D. J., Neumeier T. T., Meezan E. Serum sulfate levels in patients with cystic fibrosis. Clin Chim Acta. 1984 Sep 29;142(2):241–247. doi: 10.1016/0009-8981(84)90382-6. [DOI] [PubMed] [Google Scholar]
- Qureshi F., Jacques S. M., Johnson S. F., Johnson M. P., Hume R. F., Evans M. I., Yang S. S. Histopathology of fetal diastrophic dysplasia. Am J Med Genet. 1995 Apr 10;56(3):300–303. doi: 10.1002/ajmg.1320560317. [DOI] [PubMed] [Google Scholar]
- Schrander-Stumpel C., Havenith M., Linden E. V., Maertzdorf W., Offermans J., van der Harten J. De la Chapelle dysplasia (atelosteogenesis type II): case report and review of the literature [corrected]. Clin Dysmorphol. 1994 Oct;3(4):318–327. [PubMed] [Google Scholar]
- Sillence D. O., Kozlowski K., Rogers J. G., Sprague P. L., Cullity G. J., Osborn R. A. Atelosteogenesis: evidence for heterogeneity. Pediatr Radiol. 1987;17(2):112–118. doi: 10.1007/BF02388086. [DOI] [PubMed] [Google Scholar]
- Spranger J., Maroteaux P. The lethal osteochondrodysplasias. Adv Hum Genet. 1990;19:1-103, 331-2. doi: 10.1007/978-1-4757-9065-8_1. [DOI] [PubMed] [Google Scholar]
- Stern H. J., Graham J. M., Jr, Lachman R. S., Horton W., Bernini P. M., Spiegel P. K., Bodurtha J., Ives E. J., Bocian M., Rimoin D. L. Atelosteogenesis type III: a distinct skeletal dysplasia with features overlapping atelosteogenesis and oto-palato-digital syndrome type II. Am J Med Genet. 1990 Jun;36(2):183–195. doi: 10.1002/ajmg.1320360212. [DOI] [PubMed] [Google Scholar]
- Superti-Furga A. A defect in the metabolic activation of sulfate in a patient with achondrogenesis type IB. Am J Hum Genet. 1994 Dec;55(6):1137–1145. [PMC free article] [PubMed] [Google Scholar]
- Superti-Furga A., Hästbacka J., Wilcox W. R., Cohn D. H., van der Harten H. J., Rossi A., Blau N., Rimoin D. L., Steinmann B., Lander E. S. Achondrogenesis type IB is caused by mutations in the diastrophic dysplasia sulphate transporter gene. Nat Genet. 1996 Jan;12(1):100–102. doi: 10.1038/ng0196-100. [DOI] [PubMed] [Google Scholar]
- Walker B. A., Scott C. I., Hall J. G., Murdoch J. L., McKusick V. A. Diastrophic dwarfism. Medicine (Baltimore) 1972 Jan;51(1):41–59. doi: 10.1097/00005792-197201000-00003. [DOI] [PubMed] [Google Scholar]
- Wallis G. A. Cartilage disorders. The importance of being sulphated. Curr Biol. 1995 Mar 1;5(3):225–227. doi: 10.1016/s0960-9822(95)00044-3. [DOI] [PubMed] [Google Scholar]