Abstract
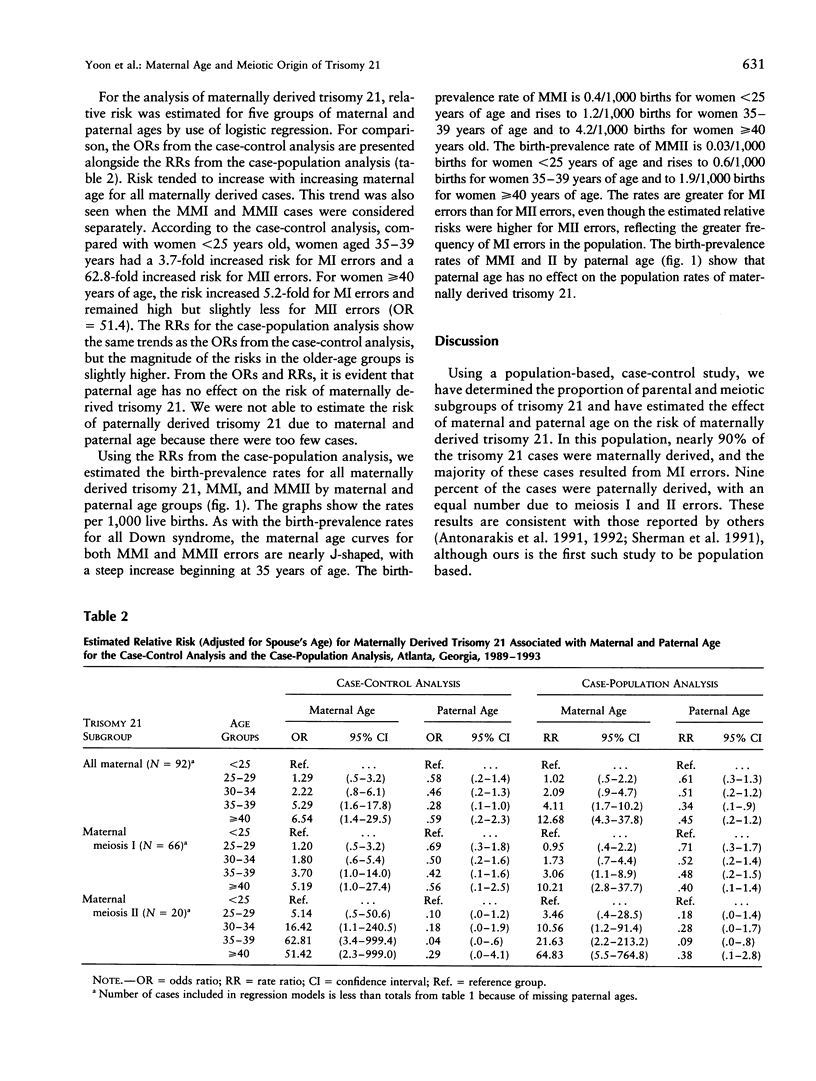
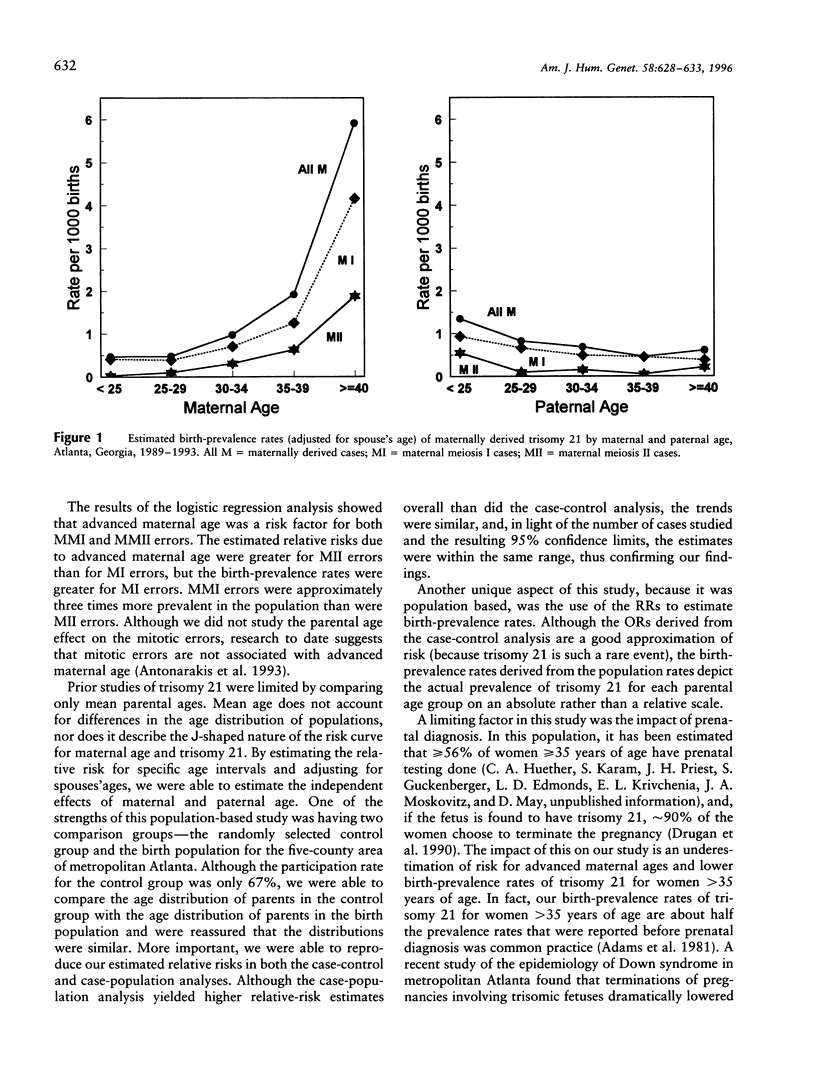
The identification of DNA polymorphisms makes it possible to classify trisomy 21 according to the parental origin and stage (meiosis I [MI], meiosis II [MII], or postzygotic mitotic) of the chromosomal error. Studying the effect of parental age on these subgroups could shed light on parental exposures and their timing. From 1989 through 1993, 170 infants with trisomy 21 and 267 randomly selected control infants were ascertained in a population-based, case-control study in metropolitan Atlanta. Blood samples for genetic studies were obtained from case infants and their parents. Using logistic regression, we independently examined the association between maternal and paternal age and subgroups of trisomy 21 defined by parental origin and meiotic stage. The distribution of trisomy 21 by origin was 86% maternal (75% MI and 25% MII), 9% paternal (50% MI and 50% MII), and 5% mitotic. Compared with women <25 years of age, women > or = 40 years old had an odds ratio of 5.2 (95% confidence interval, 1.0-27.4) for maternal MI (MMI) errors and 51.4 (95% confidence interval, 2.3-999.0) for maternal MII (MMII) errors. Birth-prevalence rates for women > or = 40 years old were 4.2/1000 births for MMI errors and 1.9/1000 for MMII errors. These results support an association between advanced maternal age and both MMI and MMII errors. The association with MI does not pinpoint the timing of the error; however, the association with MII implies that there is at least one maternal-age related mechanism acting around the time of conception.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. M., Finley S., Hansen H., Jahiel R. I., Oakley G. P., Jr, Sanger W., Wells G., Wertelecki W. Utilization of prenatal genetic diagnosis in women 35 years of age and older in the United States, 1977 to 1978. Am J Obstet Gynecol. 1981 Mar 15;139(6):673–677. doi: 10.1016/0002-9378(81)90483-x. [DOI] [PubMed] [Google Scholar]
- Antonarakis S. E., Avramopoulos D., Blouin J. L., Talbot C. C., Jr, Schinzel A. A. Mitotic errors in somatic cells cause trisomy 21 in about 4.5% of cases and are not associated with advanced maternal age. Nat Genet. 1993 Feb;3(2):146–150. doi: 10.1038/ng0293-146. [DOI] [PubMed] [Google Scholar]
- Antonarakis S. E. Parental origin of the extra chromosome in trisomy 21 as indicated by analysis of DNA polymorphisms. Down Syndrome Collaborative Group. N Engl J Med. 1991 Mar 28;324(13):872–876. doi: 10.1056/NEJM199103283241302. [DOI] [PubMed] [Google Scholar]
- Antonarakis S. E., Petersen M. B., McInnis M. G., Adelsberger P. A., Schinzel A. A., Binkert F., Pangalos C., Raoul O., Slaugenhaupt S. A., Hafez M. The meiotic stage of nondisjunction in trisomy 21: determination by using DNA polymorphisms. Am J Hum Genet. 1992 Mar;50(3):544–550. [PMC free article] [PubMed] [Google Scholar]
- Drugan A., Greb A., Johnson M. P., Krivchenia E. L., Uhlmann W. R., Moghissi K. S., Evans M. I. Determinants of parental decisions to abort for chromosome abnormalities. Prenat Diagn. 1990 Aug;10(8):483–490. doi: 10.1002/pd.1970100802. [DOI] [PubMed] [Google Scholar]
- Erickson J. D. Down syndrome, paternal age, maternal age and birth order. Ann Hum Genet. 1978 Jan;41(3):289–298. doi: 10.1111/j.1469-1809.1978.tb01896.x. [DOI] [PubMed] [Google Scholar]
- Hassold T. J., Jacobs P. A. Trisomy in man. Annu Rev Genet. 1984;18:69–97. doi: 10.1146/annurev.ge.18.120184.000441. [DOI] [PubMed] [Google Scholar]
- Hook E. B., Cross P. K., Jackson L., Pergament E., Brambati B. Maternal age-specific rates of 47,+21 and other cytogenetic abnormalities diagnosed in the first trimester of pregnancy in chorionic villus biopsy specimens: comparison with rates expected from observations at amniocentesis. Am J Hum Genet. 1988 Jun;42(6):797–807. [PMC free article] [PubMed] [Google Scholar]
- Hook E. B., Cross P. K., Schreinemachers D. M. Chromosomal abnormality rates at amniocentesis and in live-born infants. JAMA. 1983 Apr 15;249(15):2034–2038. [PubMed] [Google Scholar]
- Hook E. B., Lamson S. H. Rates of Down's syndrome at the upper extreme of maternal age--absence of a "leveling" effect and evidence for artifacts resulting from analyses of rates by five-year maternal age intervals. Am J Epidemiol. 1980 Jan;111(1):75–80. doi: 10.1093/oxfordjournals.aje.a112876. [DOI] [PubMed] [Google Scholar]
- Janerich D. T., Bracken M. B. Epidemiology of trisomy 21: a review and theoretical analysis. J Chronic Dis. 1986;39(12):1079–1093. doi: 10.1016/0021-9681(86)90141-4. [DOI] [PubMed] [Google Scholar]
- Krivchenia E., Huether C. A., Edmonds L. D., May D. S., Guckenberger S. Comparative epidemiology of Down syndrome in two United States population, 1970-1989. Am J Epidemiol. 1993 Apr 15;137(8):815–828. doi: 10.1093/oxfordjournals.aje.a116743. [DOI] [PubMed] [Google Scholar]
- Sherman S. L., Petersen M. B., Freeman S. B., Hersey J., Pettay D., Taft L., Frantzen M., Mikkelsen M., Hassold T. J. Non-disjunction of chromosome 21 in maternal meiosis I: evidence for a maternal age-dependent mechanism involving reduced recombination. Hum Mol Genet. 1994 Sep;3(9):1529–1535. doi: 10.1093/hmg/3.9.1529. [DOI] [PubMed] [Google Scholar]
- Sherman S. L., Takaesu N., Freeman S. B., Grantham M., Phillips C., Blackston R. D., Jacobs P. A., Cockwell A. E., Freeman V., Uchida I. Trisomy 21: association between reduced recombination and nondisjunction. Am J Hum Genet. 1991 Sep;49(3):608–620. [PMC free article] [PubMed] [Google Scholar]


