Abstract
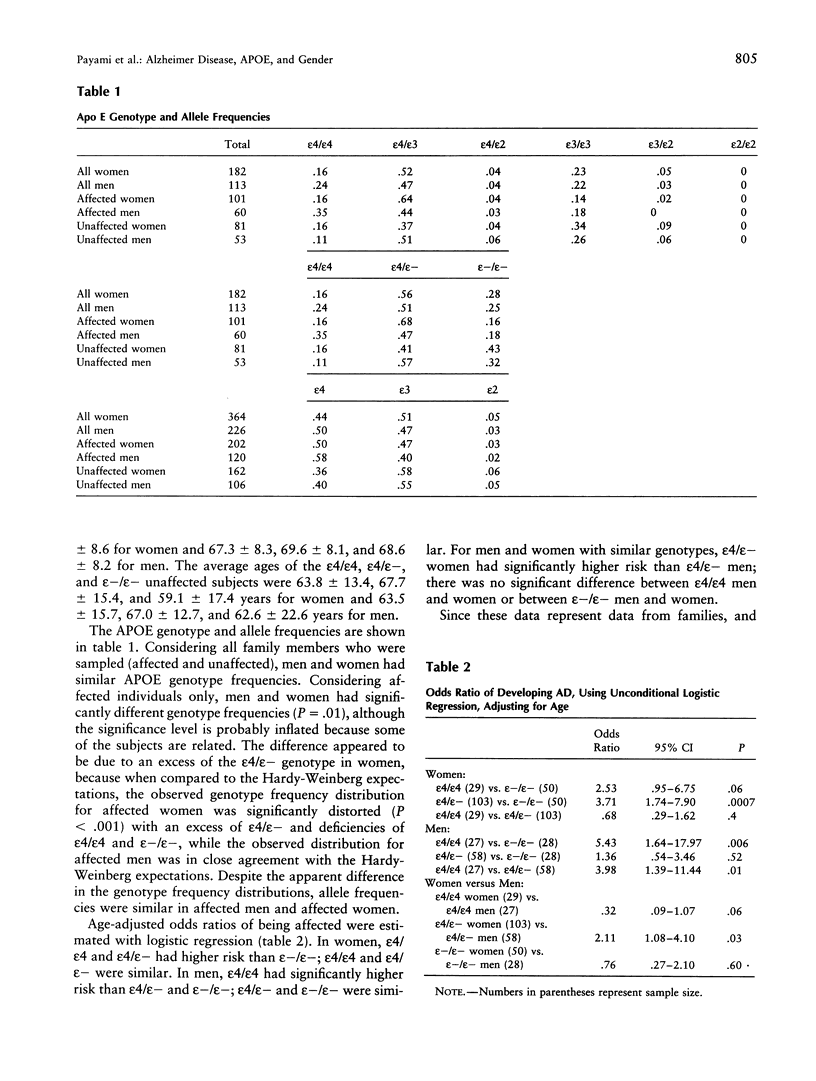
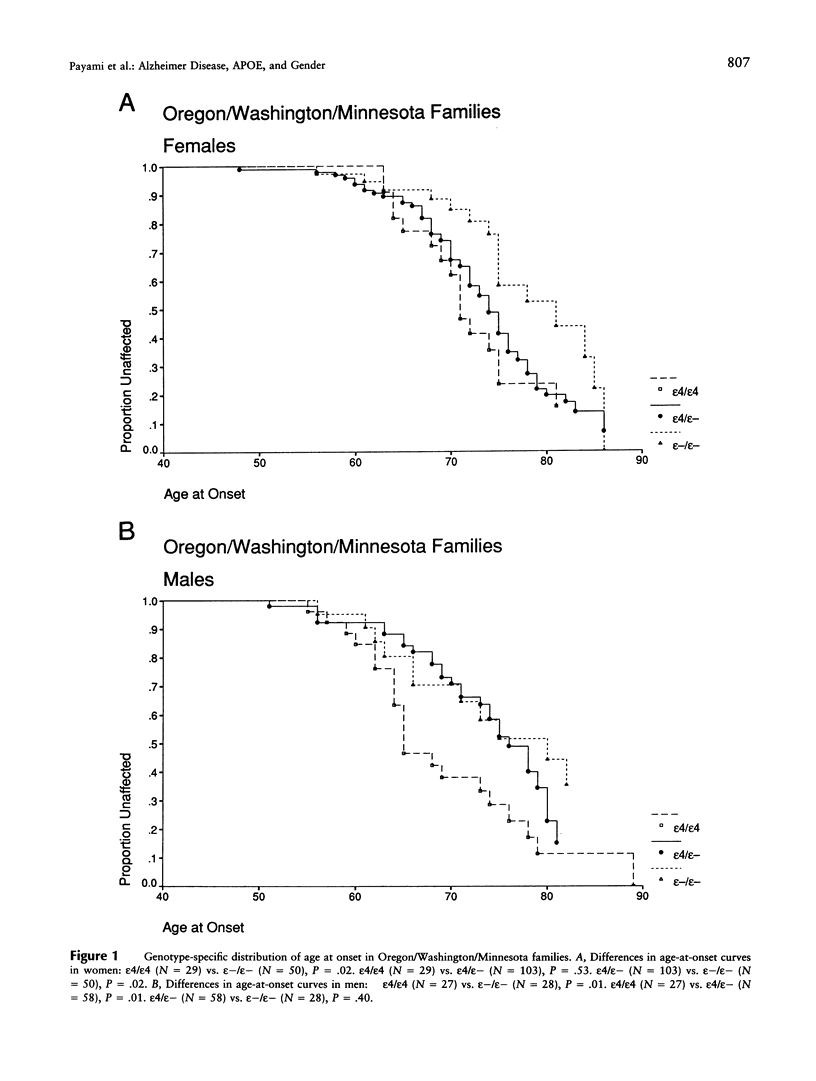
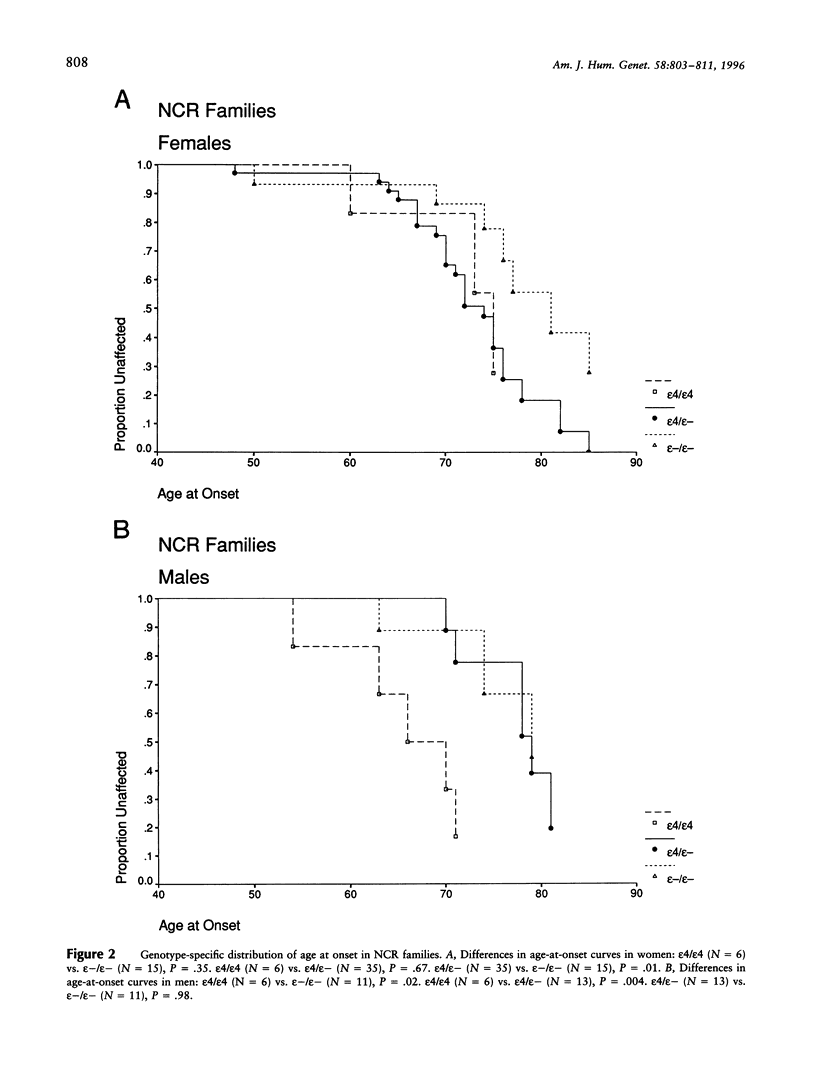
Late-onset Alzheimer disease (AD) is associated with the apolipoprotein E (APOE)-epsilon4 allele. In late-onset familial AD, women have a significantly higher risk of developing the disease than do men. The aim of this study was to determine whether the gender difference in familial AD is a function of APOE genotype. We studied 58 late-onset familial AD kindreds. Kaplan-Meier survival analysis was used to assess genotype-specific distributions of age at onset. Odds ratios were estimated by logistic regression with adjustment for age and by conditional logistic regression with stratification on families. All methods detected a significant gender difference for the epsilon4 heterozygous genotype. In women, epsilon4 heterozygotes had higher risk than those without epsilon4; there was no significant difference between epsilon4 heterozygotes and epsilon4 homozygotes. In men, epsilon4 heterozygotes had lower risk than epsilon4 homozygotes; there was not significant difference between epsilon4 heterozygotes and those without epsilon4. A direct comparison of epsilon4 heterozygous men and women revealed a significant twofold increased risk in women. We confirmed these results in 15 autopsy-confirmed AD kindreds from the National Cell Repository at Indiana University Alzheimer Disease Center. These observations are consistent with the increased incidence of familial AD in women and may be a critical clue to the role of gender in the pathogenesis of AD.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson M. K., Ooi W. L., Morgenstern H., Hafner A., Masur D., Crystal H., Frishman W. H., Fisher D., Katzman R. Women, myocardial infarction, and dementia in the very old. Neurology. 1990 Jul;40(7):1102–1106. doi: 10.1212/wnl.40.7.1102. [DOI] [PubMed] [Google Scholar]
- Bachman D. L., Wolf P. A., Linn R. T., Knoefel J. E., Cobb J. L., Belanger A. J., White L. R., D'Agostino R. B. Incidence of dementia and probable Alzheimer's disease in a general population: the Framingham Study. Neurology. 1993 Mar;43(3 Pt 1):515–519. doi: 10.1212/wnl.43.3_part_1.515. [DOI] [PubMed] [Google Scholar]
- Bachman D. L., Wolf P. A., Linn R., Knoefel J. E., Cobb J., Belanger A., D'Agostino R. B., White L. R. Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology. 1992 Jan;42(1):115–119. doi: 10.1212/wnl.42.1.115. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E., Kritz-Silverstein D. Estrogen replacement therapy and cognitive function in older women. JAMA. 1993 May 26;269(20):2637–2641. [PubMed] [Google Scholar]
- Bennett C., Crawford F., Osborne A., Diaz P., Hoyne J., Lopez R., Roques P., Duara R., Rossor M., Mullan M. Evidence that the APOE locus influences rate of disease progression in late onset familial Alzheimer's Disease but is not causative. Am J Med Genet. 1995 Feb 27;60(1):1–6. doi: 10.1002/ajmg.1320600102. [DOI] [PubMed] [Google Scholar]
- Breitner J. C., Silverman J. M., Mohs R. C., Davis K. L. Familial aggregation in Alzheimer's disease: comparison of risk among relatives of early-and late-onset cases, and among male and female relatives in successive generations. Neurology. 1988 Feb;38(2):207–212. doi: 10.1212/wnl.38.2.207. [DOI] [PubMed] [Google Scholar]
- Brenner D. E., Kukull W. A., Stergachis A., van Belle G., Bowen J. D., McCormick W. C., Teri L., Larson E. B. Postmenopausal estrogen replacement therapy and the risk of Alzheimer's disease: a population-based case-control study. Am J Epidemiol. 1994 Aug 1;140(3):262–267. doi: 10.1093/oxfordjournals.aje.a117245. [DOI] [PubMed] [Google Scholar]
- Corder E. H., Saunders A. M., Strittmatter W. J., Schmechel D. E., Gaskell P. C., Jr, Rimmler J. B., Locke P. A., Conneally P. M., Schmader K. E., Tanzi R. E. Apolipoprotein E, survival in Alzheimer's disease patients, and the competing risks of death and Alzheimer's disease. Neurology. 1995 Jul;45(7):1323–1328. doi: 10.1212/wnl.45.7.1323. [DOI] [PubMed] [Google Scholar]
- Corder E. H., Saunders A. M., Strittmatter W. J., Schmechel D. E., Gaskell P. C., Jr, Roses A. D., Pericak-Vance M. A., Small G. W., Haines J. L. The apolipoprotein E E4 allele and sex-specific risk of Alzheimer's disease. JAMA. 1995 Feb 1;273(5):373–374. [PubMed] [Google Scholar]
- Corder E. H., Saunders A. M., Strittmatter W. J., Schmechel D. E., Gaskell P. C., Small G. W., Roses A. D., Haines J. L., Pericak-Vance M. A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993 Aug 13;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Couderc R., Mahieux F., Bailleul S., Fenelon G., Mary R., Fermanian J. Prevalence of apolipoprotein E phenotypes in ischemic cerebrovascular disease. A case-control study. Stroke. 1993 May;24(5):661–664. doi: 10.1161/01.str.24.5.661. [DOI] [PubMed] [Google Scholar]
- Davignon J., Bouthillier D., Nestruck A. C., Sing C. F. Apolipoprotein E polymorphism and atherosclerosis: insight from a study in octogenarians. Trans Am Clin Climatol Assoc. 1988;99:100–110. [PMC free article] [PubMed] [Google Scholar]
- Davignon J., Gregg R. E., Sing C. F. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis. 1988 Jan-Feb;8(1):1–21. doi: 10.1161/01.atv.8.1.1. [DOI] [PubMed] [Google Scholar]
- Edwards J. K., Larson E. B., Hughes J. P., Kukull W. A. Are there clinical and epidemiological differences between familial and non-familial Alzheimer's disease? J Am Geriatr Soc. 1991 May;39(5):477–483. doi: 10.1111/j.1532-5415.1991.tb02493.x. [DOI] [PubMed] [Google Scholar]
- Evans D. A., Funkenstein H. H., Albert M. S., Scherr P. A., Cook N. R., Chown M. J., Hebert L. E., Hennekens C. H., Taylor J. O. Prevalence of Alzheimer's disease in a community population of older persons. Higher than previously reported. JAMA. 1989 Nov 10;262(18):2551–2556. [PubMed] [Google Scholar]
- Fitch N., Becker R., Heller A. The inheritance of Alzheimer's disease: a new interpretation. Ann Neurol. 1988 Jan;23(1):14–19. doi: 10.1002/ana.410230104. [DOI] [PubMed] [Google Scholar]
- Henderson B. E., Paganini-Hill A., Ross R. K. Decreased mortality in users of estrogen replacement therapy. Arch Intern Med. 1991 Jan;151(1):75–78. [PubMed] [Google Scholar]
- Hixson J. E., Vernier D. T. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990 Mar;31(3):545–548. [PubMed] [Google Scholar]
- Hofman A., Schulte W., Tanja T. A., van Duijn C. M., Haaxma R., Lameris A. J., Otten V. M., Saan R. J. History of dementia and Parkinson's disease in 1st-degree relatives of patients with Alzheimer's disease. Neurology. 1989 Dec;39(12):1589–1592. doi: 10.1212/wnl.39.12.1589. [DOI] [PubMed] [Google Scholar]
- Honjo H., Ogino Y., Naitoh K., Urabe M., Kitawaki J., Yasuda J., Yamamoto T., Ishihara S., Okada H., Yonezawa T. In vivo effects by estrone sulfate on the central nervous system-senile dementia (Alzheimer's type). J Steroid Biochem. 1989;34(1-6):521–525. doi: 10.1016/0022-4731(89)90137-4. [DOI] [PubMed] [Google Scholar]
- Jarvik G. P., Wijsman E. M., Kukull W. A., Schellenberg G. D., Yu C., Larson E. B. Interactions of apolipoprotein E genotype, total cholesterol level, age, and sex in prediction of Alzheimer's disease: a case-control study. Neurology. 1995 Jun;45(6):1092–1096. doi: 10.1212/wnl.45.6.1092. [DOI] [PubMed] [Google Scholar]
- Kamino K., Orr H. T., Payami H., Wijsman E. M., Alonso M. E., Pulst S. M., Anderson L., O'dahl S., Nemens E., White J. A. Linkage and mutational analysis of familial Alzheimer disease kindreds for the APP gene region. Am J Hum Genet. 1992 Nov;51(5):998–1014. [PMC free article] [PubMed] [Google Scholar]
- Kampen D. L., Sherwin B. B. Estrogen use and verbal memory in healthy postmenopausal women. Obstet Gynecol. 1994 Jun;83(6):979–983. doi: 10.1097/00006250-199406000-00017. [DOI] [PubMed] [Google Scholar]
- Katzman R., Aronson M., Fuld P., Kawas C., Brown T., Morgenstern H., Frishman W., Gidez L., Eder H., Ooi W. L. Development of dementing illnesses in an 80-year-old volunteer cohort. Ann Neurol. 1989 Apr;25(4):317–324. doi: 10.1002/ana.410250402. [DOI] [PubMed] [Google Scholar]
- Kervinen K., Savolainen M. J., Salokannel J., Hynninen A., Heikkinen J., Ehnholm C., Koistinen M. J., Kesäniemi Y. A. Apolipoprotein E and B polymorphisms--longevity factors assessed in nonagenarians. Atherosclerosis. 1994 Jan;105(1):89–95. doi: 10.1016/0021-9150(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Kokmen E., Chandra V., Schoenberg B. S. Trends in incidence of dementing illness in Rochester, Minnesota, in three quinquennial periods, 1960-1974. Neurology. 1988 Jun;38(6):975–980. doi: 10.1212/wnl.38.6.975. [DOI] [PubMed] [Google Scholar]
- Levy-Lahad E., Wasco W., Poorkaj P., Romano D. M., Oshima J., Pettingell W. H., Yu C. E., Jondro P. D., Schmidt S. D., Wang K. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science. 1995 Aug 18;269(5226):973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E. M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984 Jul;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Paganini-Hill A., Henderson V. W. Estrogen deficiency and risk of Alzheimer's disease in women. Am J Epidemiol. 1994 Aug 1;140(3):256–261. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- Payami H., Kaye J., Heston L. L., Bird T. D., Schellenberg G. D. Apolipoprotein E genotype and Alzheimer's disease. Lancet. 1993 Sep 18;342(8873):738–738. [PubMed] [Google Scholar]
- Payami H., Montee K. R., Kaye J. A., Bird T. D., Yu C. E., Wijsman E. M., Schellenberg G. D. Alzheimer's disease, apolipoprotein E4, and gender. JAMA. 1994 May 4;271(17):1316–1317. [PubMed] [Google Scholar]
- Payami H., Montee K., Grimslid H., Shattuc S., Kaye J. Increased risk of familial late-onset Alzheimer's disease in women. Neurology. 1996 Jan;46(1):126–129. doi: 10.1212/wnl.46.1.126. [DOI] [PubMed] [Google Scholar]
- Payami H., Montee K., Kaye J. Evidence for familial factors that protect against dementia and outweigh the effect of increasing age. Am J Hum Genet. 1994 Apr;54(4):650–657. [PMC free article] [PubMed] [Google Scholar]
- Payne M. N., Green E., Walker M. R., Beattie J. M., Murray R. G., Jones A. F. Apolipoprotein epsilon 4 and coronary artery disease. Lancet. 1992 Nov 28;340(8831):1350–1350. [PubMed] [Google Scholar]
- Pedro-Botet J., Sentí M., Nogués X., Rubiés-Prat J., Roquer J., D'Olhaberriague L., Olivé J. Lipoprotein and apolipoprotein profile in men with ischemic stroke. Role of lipoprotein(a), triglyceride-rich lipoproteins, and apolipoprotein E polymorphism. Stroke. 1992 Nov;23(11):1556–1562. doi: 10.1161/01.str.23.11.1556. [DOI] [PubMed] [Google Scholar]
- Poirier J. Apolipoprotein E in animal models of CNS injury and in Alzheimer's disease. Trends Neurosci. 1994 Dec;17(12):525–530. doi: 10.1016/0166-2236(94)90156-2. [DOI] [PubMed] [Google Scholar]
- Poirier J., Davignon J., Bouthillier D., Kogan S., Bertrand P., Gauthier S. Apolipoprotein E polymorphism and Alzheimer's disease. Lancet. 1993 Sep 18;342(8873):697–699. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- Rao V. S., van Duijn C. M., Connor-Lacke L., Cupples L. A., Growdon J. H., Farrer L. A. Multiple etiologies for Alzheimer disease are revealed by segregation analysis. Am J Hum Genet. 1994 Nov;55(5):991–1000. [PMC free article] [PubMed] [Google Scholar]
- Reilly S. L., Ferrell R. E., Sing C. F. The gender-specific apolipoprotein E genotype influence on the distribution of plasma lipids and apolipoproteins in the population of Rochester, MN. III. Correlations and covariances. Am J Hum Genet. 1994 Nov;55(5):1001–1018. [PMC free article] [PubMed] [Google Scholar]
- Robinson D., Friedman L., Marcus R., Tinklenberg J., Yesavage J. Estrogen replacement therapy and memory in older women. J Am Geriatr Soc. 1994 Sep;42(9):919–922. doi: 10.1111/j.1532-5415.1994.tb06580.x. [DOI] [PubMed] [Google Scholar]
- Rocca W. A., Hofman A., Brayne C., Breteler M. M., Clarke M., Copeland J. R., Dartigues J. F., Engedal K., Hagnell O., Heeren T. J. Frequency and distribution of Alzheimer's disease in Europe: a collaborative study of 1980-1990 prevalence findings. The EURODEM-Prevalence Research Group. Ann Neurol. 1991 Sep;30(3):381–390. doi: 10.1002/ana.410300310. [DOI] [PubMed] [Google Scholar]
- Saunders A. M., Strittmatter W. J., Schmechel D., George-Hyslop P. H., Pericak-Vance M. A., Joo S. H., Rosi B. L., Gusella J. F., Crapper-MacLachlan D. R., Alberts M. J. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993 Aug;43(8):1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Schellenberg G. D., Payami H., Wijsman E. M., Orr H. T., Goddard K. A., Anderson L., Nemens E., White J. A., Alonso M. E., Ball M. J. Chromosome 14 and late-onset familial Alzheimer disease (FAD). Am J Hum Genet. 1993 Sep;53(3):619–628. [PMC free article] [PubMed] [Google Scholar]
- Schoenberg B. S., Kokmen E., Okazaki H. Alzheimer's disease and other dementing illnesses in a defined United States population: incidence rates and clinical features. Ann Neurol. 1987 Dec;22(6):724–729. doi: 10.1002/ana.410220608. [DOI] [PubMed] [Google Scholar]
- Schächter F., Faure-Delanef L., Guénot F., Rouger H., Froguel P., Lesueur-Ginot L., Cohen D. Genetic associations with human longevity at the APOE and ACE loci. Nat Genet. 1994 Jan;6(1):29–32. doi: 10.1038/ng0194-29. [DOI] [PubMed] [Google Scholar]
- Silverman J. M., Raiford K., Edland S., Fillenbaum G., Morris J. C., Clark C. M., Kukull W., Heyman A. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part VI. Family history assessment: a multicenter study of first-degree relatives of Alzheimer's disease probands and nondemented spouse controls. Neurology. 1994 Jul;44(7):1253–1259. doi: 10.1212/wnl.44.7.1253. [DOI] [PubMed] [Google Scholar]
- Tsai M. S., Tangalos E. G., Petersen R. C., Smith G. E., Schaid D. J., Kokmen E., Ivnik R. J., Thibodeau S. N. Apolipoprotein E: risk factor for Alzheimer disease. Am J Hum Genet. 1994 Apr;54(4):643–649. [PMC free article] [PubMed] [Google Scholar]
- Wilson P. W., Myers R. H., Larson M. G., Ordovas J. M., Wolf P. A., Schaefer E. J. Apolipoprotein E alleles, dyslipidemia, and coronary heart disease. The Framingham Offspring Study. JAMA. 1994 Dec 7;272(21):1666–1671. [PubMed] [Google Scholar]
- van Bockxmeer F. M., Mamotte C. D. Apolipoprotein epsilon 4 homozygosity in young men with coronary heart disease. Lancet. 1992 Oct 10;340(8824):879–880. doi: 10.1016/0140-6736(92)93288-x. [DOI] [PubMed] [Google Scholar]
- van Duijn C. M., Farrer L. A., Cupples L. A., Hofman A. Genetic transmission of Alzheimer's disease among families in a Dutch population based study. J Med Genet. 1993 Aug;30(8):640–646. doi: 10.1136/jmg.30.8.640. [DOI] [PMC free article] [PubMed] [Google Scholar]


