Abstract
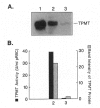
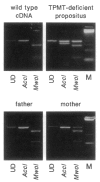
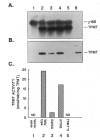
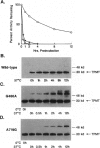
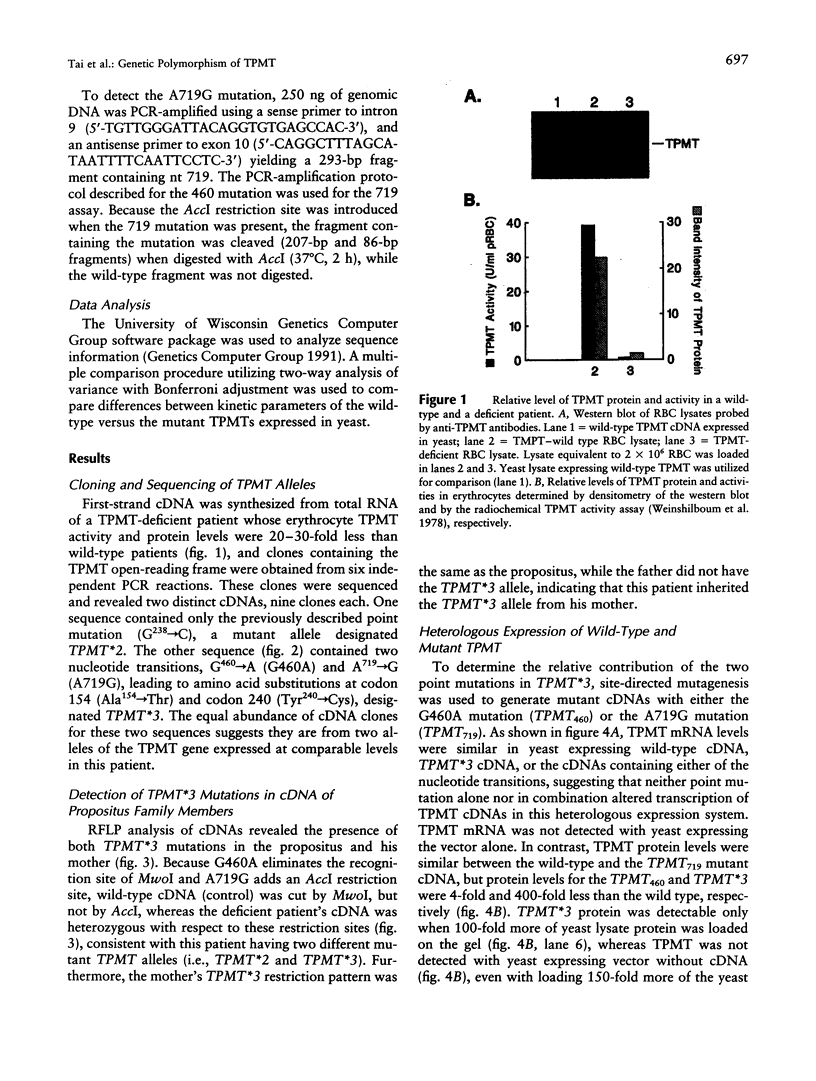
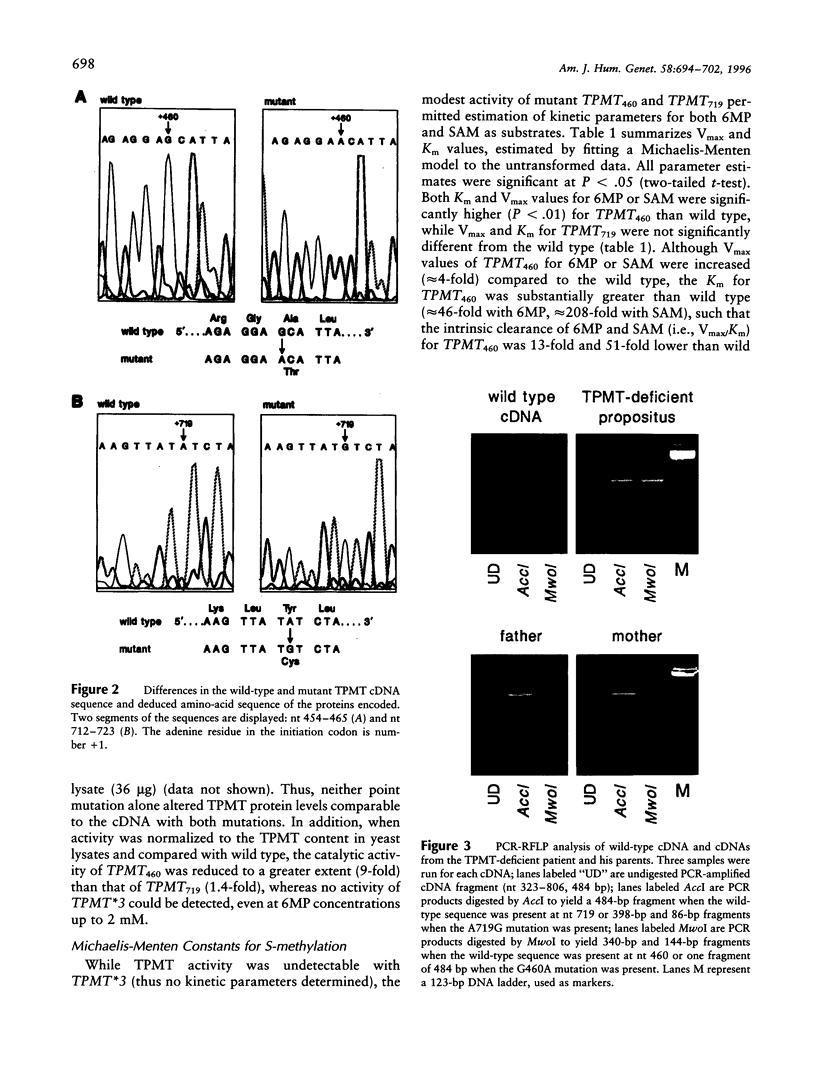
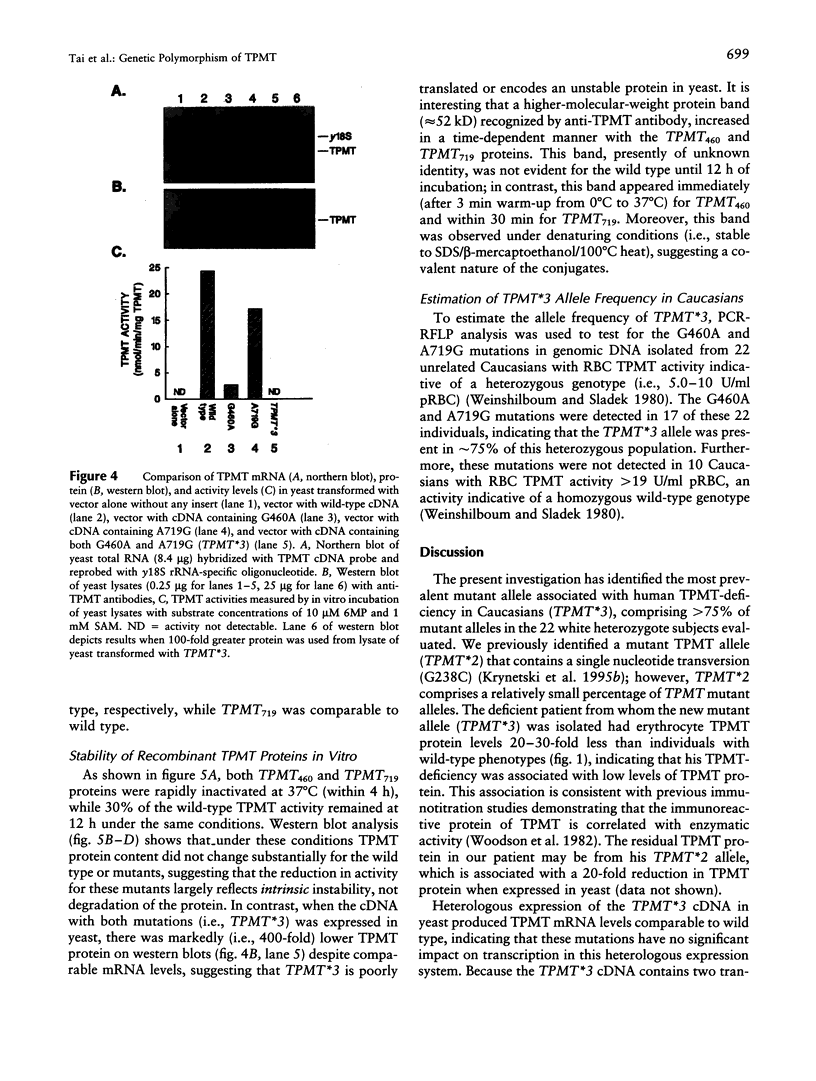
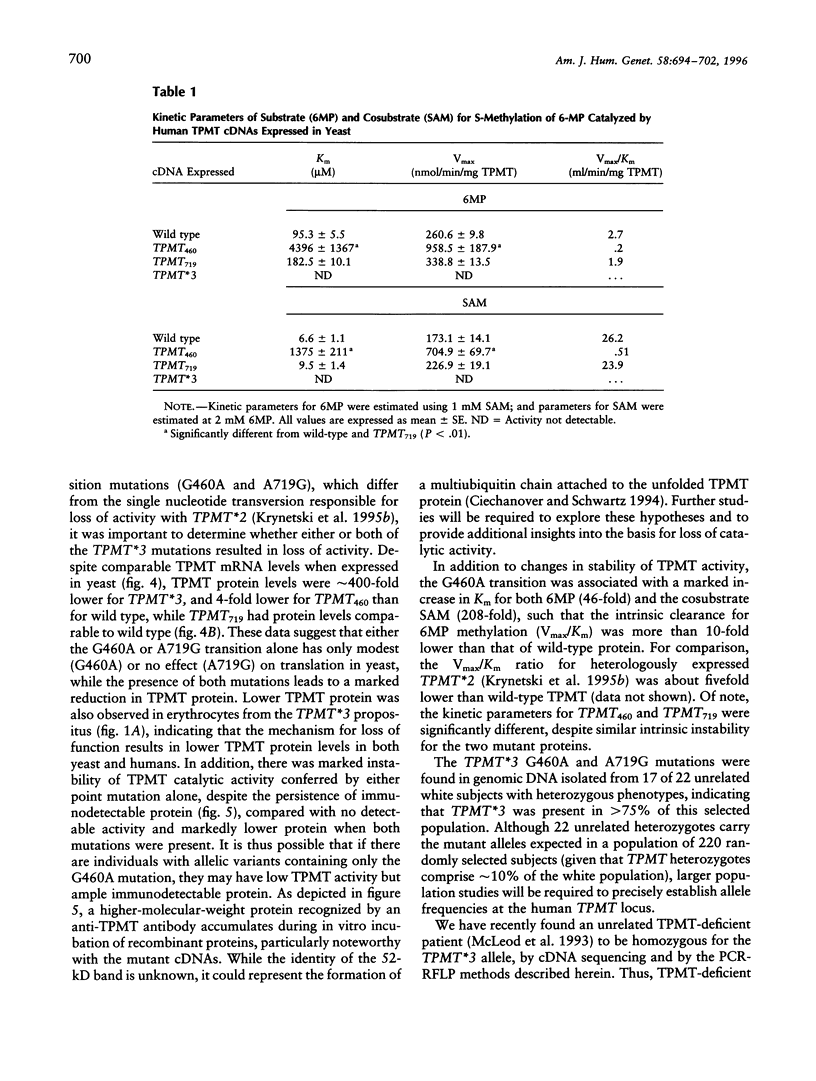
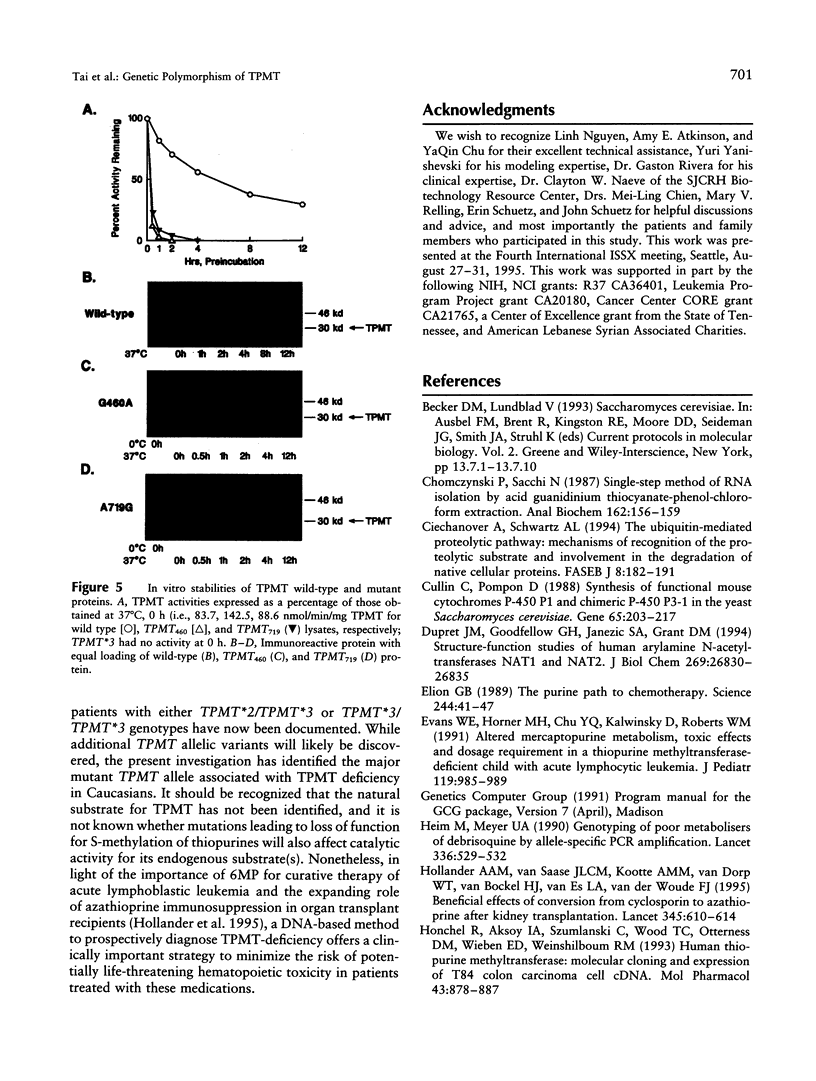
The autosomal recessive trait of thiopurine S-methytransferase (TPMT) deficiency is associated with severe hematopoietic toxicity when patients are treated with standard doses of mercaptopurine, azathioprine, or thioguanine. To define the molecular mechanism of this genetic polymorphism, we cloned and characterized the cDNA of a TPMT-deficient patient, which revealed a novel mutant allele (TPMT*3) containing two nucleotide transitions (G460-->A and A719-->G) producing amino acid changes at codons 154 (Ala-->Thr) and 240 (Tyr--> Cys), differing from the rare mutant TPMT allele we previously identified (i.e., TPMT*2 with only G238-->C). Site-directed mutagenesis and heterologous expression established that either TPMT*3 mutation alone leads to a reduction in catalytic activity (G460-->A, ninefold reduction; A719-->G, 1.4-fold reduction), while the presence of both mutations leads to complete loss of activity. Using mutation specific PCR-RFLP analysis, the TPMT*3 allele was detected in genomic DNA from approximately 75 percent of unrelated white subjects with heterozygous phenotypes, indicating that TPMT*3 is the most prevalent mutant allele associated with TPMT-deficiency in Caucasians.
Full text
PDF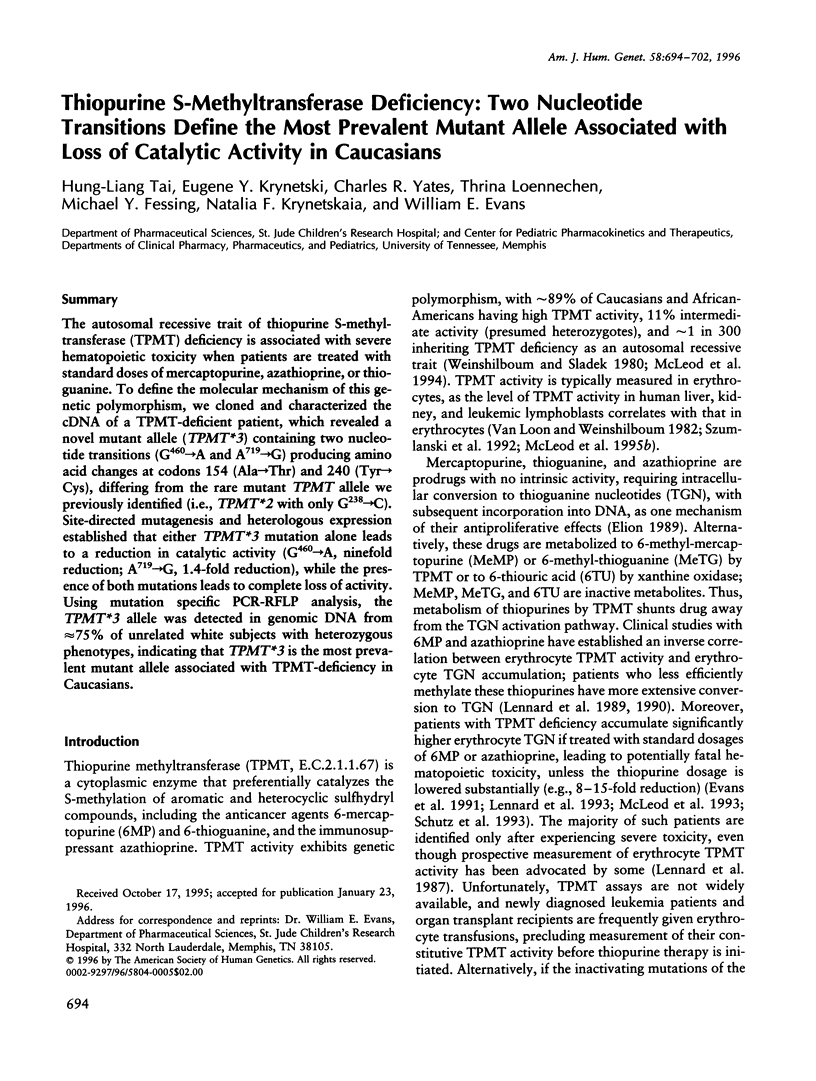



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Ciechanover A., Schwartz A. L. The ubiquitin-mediated proteolytic pathway: mechanisms of recognition of the proteolytic substrate and involvement in the degradation of native cellular proteins. FASEB J. 1994 Feb;8(2):182–191. doi: 10.1096/fasebj.8.2.8119489. [DOI] [PubMed] [Google Scholar]
- Cullin C., Pompon D. Synthesis of functional mouse cytochromes P-450 P1 and chimeric P-450 P3-1 in the yeast Saccharomyces cerevisiae. Gene. 1988 May 30;65(2):203–217. doi: 10.1016/0378-1119(88)90457-x. [DOI] [PubMed] [Google Scholar]
- Dupret J. M., Goodfellow G. H., Janezic S. A., Grant D. M. Structure-function studies of human arylamine N-acetyltransferases NAT1 and NAT2. Functional analysis of recombinant NAT1/NAT2 chimeras expressed in Escherichia coli. J Biol Chem. 1994 Oct 28;269(43):26830–26835. [PubMed] [Google Scholar]
- Elion G. B. The purine path to chemotherapy. Science. 1989 Apr 7;244(4900):41–47. doi: 10.1126/science.2649979. [DOI] [PubMed] [Google Scholar]
- Evans W. E., Horner M., Chu Y. Q., Kalwinsky D., Roberts W. M. Altered mercaptopurine metabolism, toxic effects, and dosage requirement in a thiopurine methyltransferase-deficient child with acute lymphocytic leukemia. J Pediatr. 1991 Dec;119(6):985–989. doi: 10.1016/s0022-3476(05)83063-x. [DOI] [PubMed] [Google Scholar]
- Heim M., Meyer U. A. Genotyping of poor metabolisers of debrisoquine by allele-specific PCR amplification. Lancet. 1990 Sep 1;336(8714):529–532. doi: 10.1016/0140-6736(90)92086-w. [DOI] [PubMed] [Google Scholar]
- Hollander A. A., van Saase J. L., Kootte A. M., van Dorp W. T., van Bockel H. J., van Es L. A., van der Woude F. J. Beneficial effects of conversion from cyclosporin to azathioprine after kidney transplantation. Lancet. 1995 Mar 11;345(8950):610–614. doi: 10.1016/s0140-6736(95)90520-0. [DOI] [PubMed] [Google Scholar]
- Honchel R., Aksoy I. A., Szumlanski C., Wood T. C., Otterness D. M., Wieben E. D., Weinshilboum R. M. Human thiopurine methyltransferase: molecular cloning and expression of T84 colon carcinoma cell cDNA. Mol Pharmacol. 1993 Jun;43(6):878–887. [PubMed] [Google Scholar]
- Krynetski E. Y., Krynetskaia N. F., Yanishevski Y., Evans W. E. Methylation of mercaptopurine, thioguanine, and their nucleotide metabolites by heterologously expressed human thiopurine S-methyltransferase. Mol Pharmacol. 1995 Jun;47(6):1141–1147. [PubMed] [Google Scholar]
- Krynetski E. Y., Schuetz J. D., Galpin A. J., Pui C. H., Relling M. V., Evans W. E. A single point mutation leading to loss of catalytic activity in human thiopurine S-methyltransferase. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):949–953. doi: 10.1073/pnas.92.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lennard L., Gibson B. E., Nicole T., Lilleyman J. S. Congenital thiopurine methyltransferase deficiency and 6-mercaptopurine toxicity during treatment for acute lymphoblastic leukaemia. Arch Dis Child. 1993 Nov;69(5):577–579. doi: 10.1136/adc.69.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard L., Lilleyman J. S., Van Loon J., Weinshilboum R. M. Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia. Lancet. 1990 Jul 28;336(8709):225–229. doi: 10.1016/0140-6736(90)91745-v. [DOI] [PubMed] [Google Scholar]
- Lennard L., Van Loon J. A., Lilleyman J. S., Weinshilboum R. M. Thiopurine pharmacogenetics in leukemia: correlation of erythrocyte thiopurine methyltransferase activity and 6-thioguanine nucleotide concentrations. Clin Pharmacol Ther. 1987 Jan;41(1):18–25. doi: 10.1038/clpt.1987.4. [DOI] [PubMed] [Google Scholar]
- Lennard L., Van Loon J. A., Weinshilboum R. M. Pharmacogenetics of acute azathioprine toxicity: relationship to thiopurine methyltransferase genetic polymorphism. Clin Pharmacol Ther. 1989 Aug;46(2):149–154. doi: 10.1038/clpt.1989.119. [DOI] [PubMed] [Google Scholar]
- McLeod H. L., Krynetski E. Y., Wilimas J. A., Evans W. E. Higher activity of polymorphic thiopurine S-methyltransferase in erythrocytes from neonates compared to adults. Pharmacogenetics. 1995 Oct;5(5):281–286. doi: 10.1097/00008571-199510000-00003. [DOI] [PubMed] [Google Scholar]
- McLeod H. L., Lin J. S., Scott E. P., Pui C. H., Evans W. E. Thiopurine methyltransferase activity in American white subjects and black subjects. Clin Pharmacol Ther. 1994 Jan;55(1):15–20. doi: 10.1038/clpt.1994.4. [DOI] [PubMed] [Google Scholar]
- McLeod H. L., Miller D. R., Evans W. E. Azathioprine-induced myelosuppression in thiopurine methyltransferase deficient heart transplant recipient. Lancet. 1993 May 1;341(8853):1151–1151. doi: 10.1016/0140-6736(93)93168-z. [DOI] [PubMed] [Google Scholar]
- McLeod H. L., Relling M. V., Liu Q., Pui C. H., Evans W. E. Polymorphic thiopurine methyltransferase in erythrocytes is indicative of activity in leukemic blasts from children with acute lymphoblastic leukemia. Blood. 1995 Apr 1;85(7):1897–1902. [PubMed] [Google Scholar]
- Schmitt M. E., Brown T. A., Trumpower B. L. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 1990 May 25;18(10):3091–3092. doi: 10.1093/nar/18.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütz E., Gummert J., Mohr F., Oellerich M. Azathioprine-induced myelosuppression in thiopurine methyltransferase deficient heart transplant recipient. Lancet. 1993 Feb 13;341(8842):436–436. doi: 10.1016/0140-6736(93)93028-y. [DOI] [PubMed] [Google Scholar]
- Szumlanski C. L., Honchel R., Scott M. C., Weinshilboum R. M. Human liver thiopurine methyltransferase pharmacogenetics: biochemical properties, liver-erythrocyte correlation and presence of isozymes. Pharmacogenetics. 1992 Aug;2(4):148–159. [PubMed] [Google Scholar]
- Van Loon J. A., Weinshilboum R. M. Thiopurine methyltransferase biochemical genetics: human lymphocyte activity. Biochem Genet. 1982 Aug;20(7-8):637–658. doi: 10.1007/BF00483962. [DOI] [PubMed] [Google Scholar]
- Weinshilboum R. M., Raymond F. A., Pazmiño P. A. Human erythrocyte thiopurine methyltransferase: radiochemical microassay and biochemical properties. Clin Chim Acta. 1978 May 2;85(3):323–333. doi: 10.1016/0009-8981(78)90311-x. [DOI] [PubMed] [Google Scholar]
- Weinshilboum R. M., Sladek S. L. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet. 1980 Sep;32(5):651–662. [PMC free article] [PubMed] [Google Scholar]
- Woodson L. C., Dunnette J. H., Weinshilboum R. M. Pharmacogenetics of human thiopurine methyltransferase: kidney-erythrocyte correlation and immunotitration studies. J Pharmacol Exp Ther. 1982 Jul;222(1):174–181. [PubMed] [Google Scholar]