Abstract
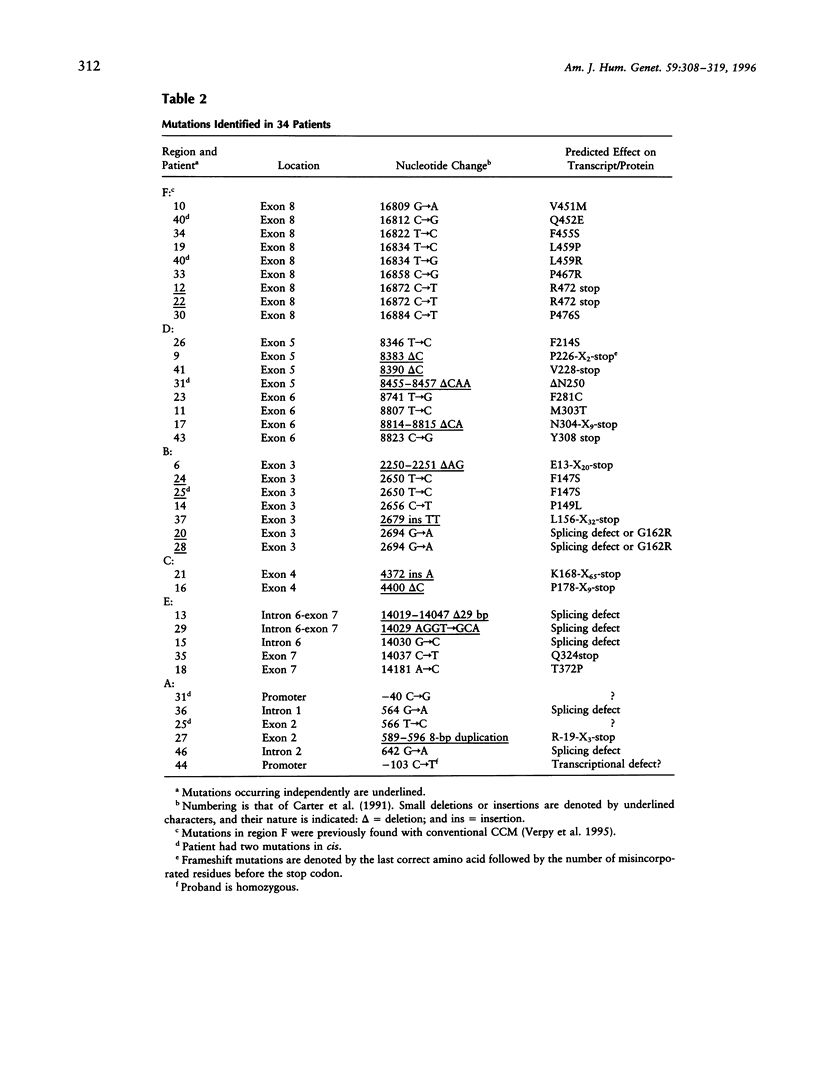
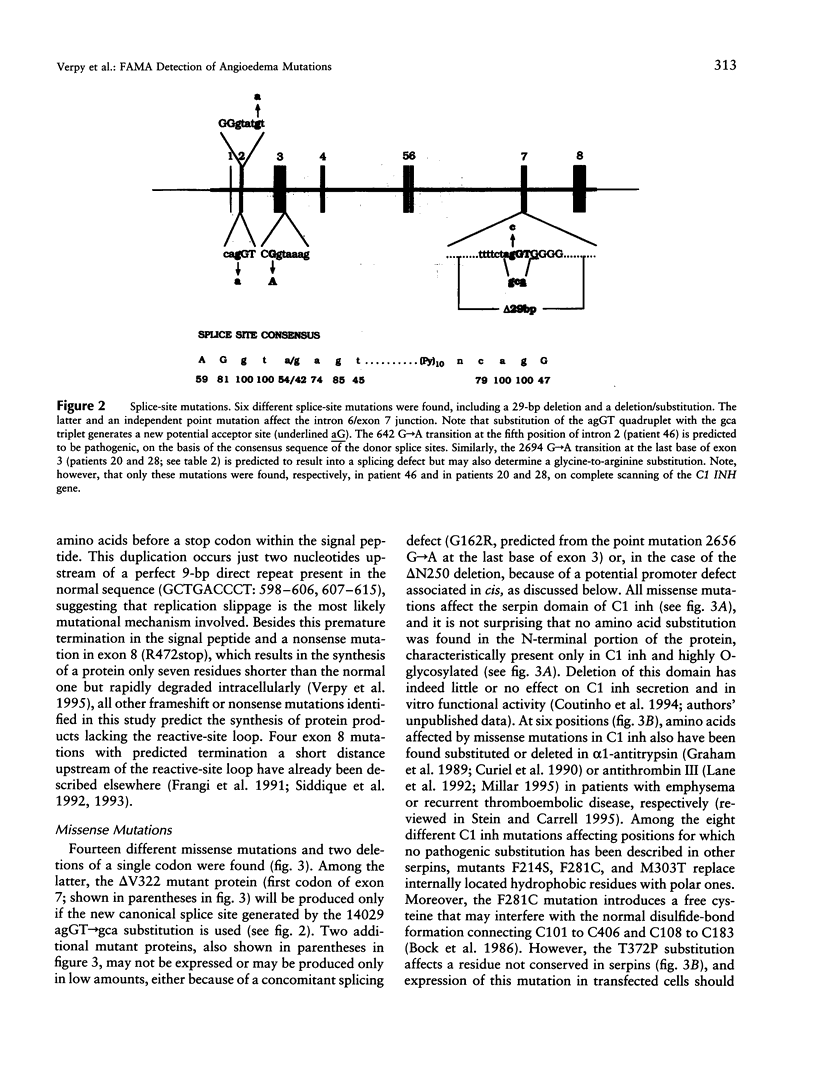
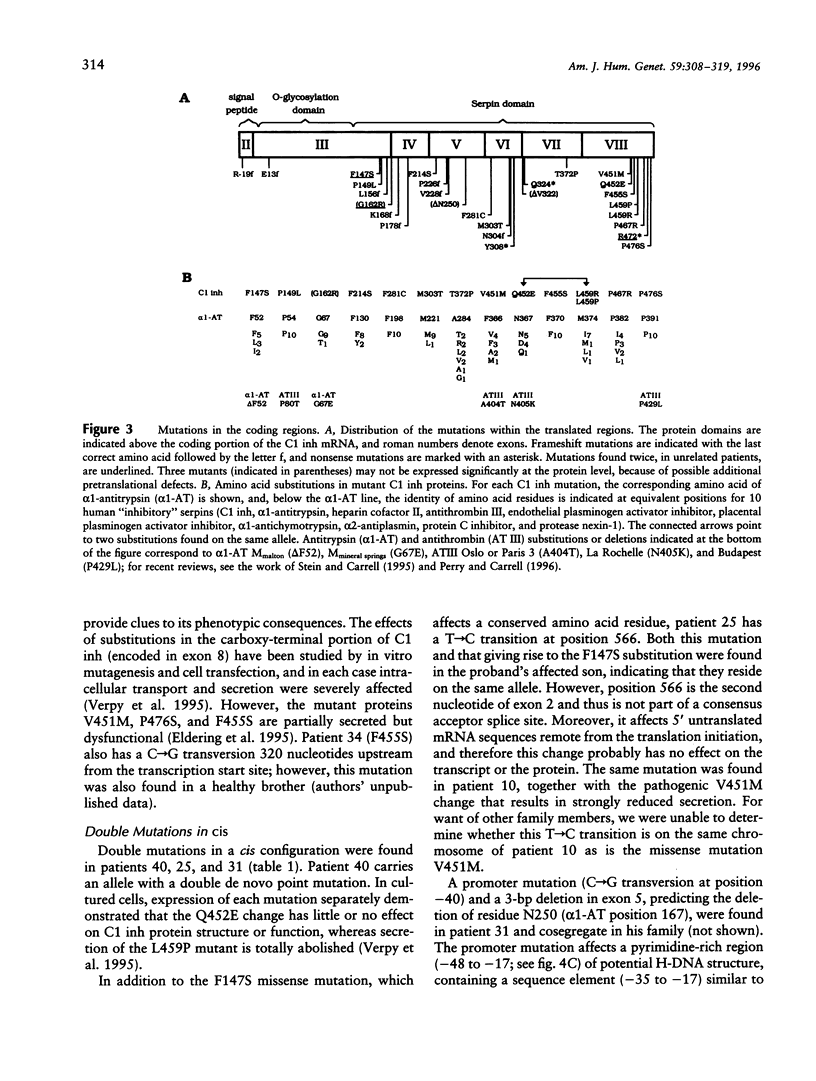
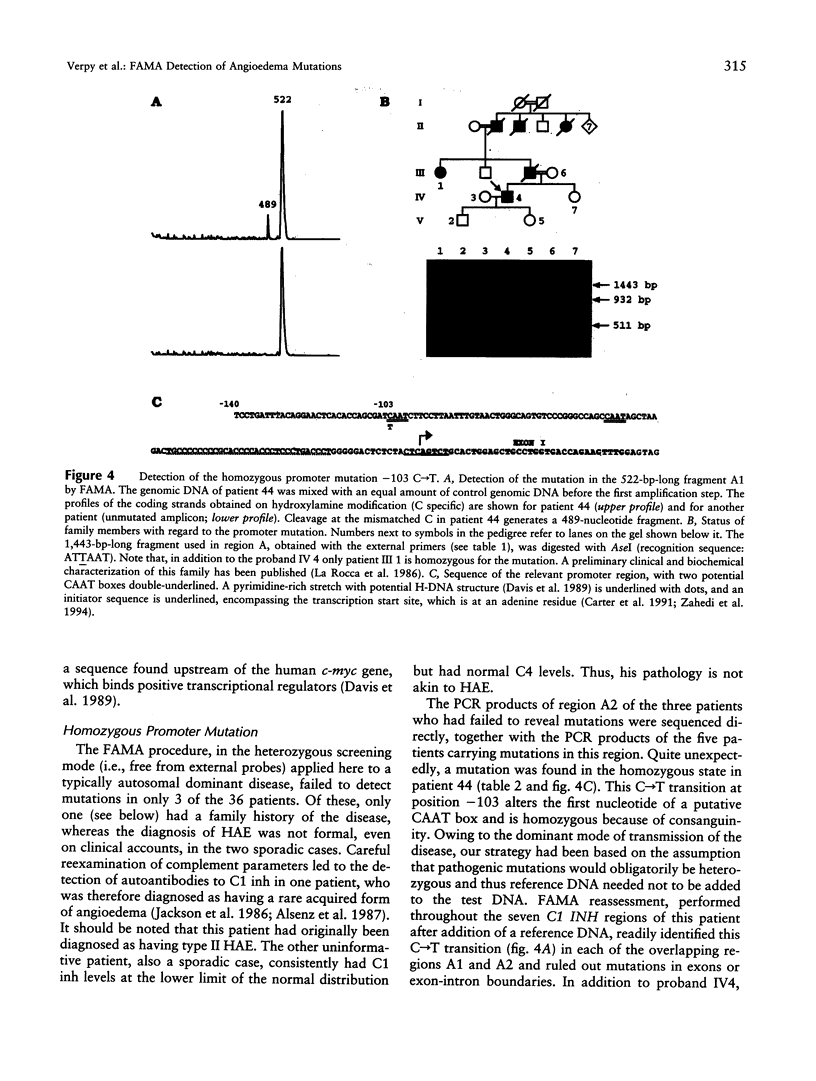
A complete mutational scan of the gene coding for the serpin C1 inhibitor, comprising all eight exons and adjacent intron sequences and 550 bp preceding the transcription start site, was rapidly accomplished in 36 unrelated angioedema patients by using fluorescence-assisted mismatch analysis (FAMA). Mutations accounting for C1 inhibitor deficiency were identified in every one of 34 patients, with two failures turning out to be spurious cases. Two new substitution dimorphisms were also detected in introns. Changes affecting the C1 inhibitor protein, distributed throughout the seven coding exons, provide new insights into the molecular pathology of serpins. Six different splice-site and two promoter mutations were also found. Among the latter, a C-->T transition within one of two putative CAAT boxes of this TATA-less promoter, the sole idiomorphic nucleotide change in this kindred, was found homozygous in the proband, at variance with the dominant mode of transmission observed for structural mutations. FAMA, in the chemical probes configuration used in this study, is a rapid and robust mutation-scanning procedure, applicable to large DNA segments or transcripts and proved capable of 100% detection. Moreover, it provides accurate positional information--and hence recognition of multiple substitutions, precise relationship with those already known, and often immediate identification of the nucleotide change.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bock S. C., Skriver K., Nielsen E., Thøgersen H. C., Wiman B., Donaldson V. H., Eddy R. L., Marrinan J., Radziejewska E., Huber R. Human C1 inhibitor: primary structure, cDNA cloning, and chromosomal localization. Biochemistry. 1986 Jul 29;25(15):4292–4301. doi: 10.1021/bi00363a018. [DOI] [PubMed] [Google Scholar]
- Carter P. E., Duponchel C., Tosi M., Fothergill J. E. Complete nucleotide sequence of the gene for human C1 inhibitor with an unusually high density of Alu elements. Eur J Biochem. 1991 Apr 23;197(2):301–308. doi: 10.1111/j.1432-1033.1991.tb15911.x. [DOI] [PubMed] [Google Scholar]
- Cotton R. G., Campbell R. D. Chemical reactivity of matched cytosine and thymine bases near mismatched and unmatched bases in a heteroduplex between DNA strands with multiple differences. Nucleic Acids Res. 1989 Jun 12;17(11):4223–4233. doi: 10.1093/nar/17.11.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton R. G. Current methods of mutation detection. Mutat Res. 1993 Jan;285(1):125–144. doi: 10.1016/0027-5107(93)90060-s. [DOI] [PubMed] [Google Scholar]
- Coutinho M., Aulak K. S., Davis A. E., 3rd Functional analysis of the serpin domain of C1 inhibitor. J Immunol. 1994 Oct 15;153(8):3648–3654. [PubMed] [Google Scholar]
- Crossley M., Brownlee G. G. Disruption of a C/EBP binding site in the factor IX promoter is associated with haemophilia B. Nature. 1990 May 31;345(6274):444–446. doi: 10.1038/345444a0. [DOI] [PubMed] [Google Scholar]
- Crossley M., Ludwig M., Stowell K. M., De Vos P., Olek K., Brownlee G. G. Recovery from hemophilia B Leyden: an androgen-responsive element in the factor IX promoter. Science. 1992 Jul 17;257(5068):377–379. doi: 10.1126/science.1631558. [DOI] [PubMed] [Google Scholar]
- Curiel D. T., Vogelmeier C., Hubbard R. C., Stier L. E., Crystal R. G. Molecular basis of alpha 1-antitrypsin deficiency and emphysema associated with the alpha 1-antitrypsin Mmineral springs allele. Mol Cell Biol. 1990 Jan;10(1):47–56. doi: 10.1128/mcb.10.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. E., 3rd, Aulak K., Parad R. B., Stecklein H. P., Eldering E., Hack C. E., Kramer J., Strunk R. C., Bissler J., Rosen F. S. C1 inhibitor hinge region mutations produce dysfunction by different mechanisms. Nat Genet. 1992 Aug;1(5):354–358. doi: 10.1038/ng0892-354. [DOI] [PubMed] [Google Scholar]
- Davis A. E., 3rd C1 inhibitor and hereditary angioneurotic edema. Annu Rev Immunol. 1988;6:595–628. doi: 10.1146/annurev.iy.06.040188.003115. [DOI] [PubMed] [Google Scholar]
- Davis T. L., Firulli A. B., Kinniburgh A. J. Ribonucleoprotein and protein factors bind to an H-DNA-forming c-myc DNA element: possible regulators of the c-myc gene. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9682–9686. doi: 10.1073/pnas.86.24.9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldering E., Verpy E., Roem D., Meo T., Tosi M. COOH-terminal substitutions in the serpin C1 inhibitor that cause loop overinsertion and subsequent multimerization. J Biol Chem. 1995 Feb 10;270(6):2579–2587. doi: 10.1074/jbc.270.6.2579. [DOI] [PubMed] [Google Scholar]
- Frangi D., Cicardi M., Sica A., Colotta F., Agostoni A., Davis A. E., 3rd Nonsense mutations affect C1 inhibitor messenger RNA levels in patients with type I hereditary angioneurotic edema. J Clin Invest. 1991 Sep;88(3):755–759. doi: 10.1172/JCI115373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A., Kalsheker N. A., Newton C. R., Bamforth F. J., Powell S. J., Markham A. F. Molecular characterisation of three alpha-1-antitrypsin deficiency variants: proteinase inhibitor (Pi) nullcardiff (Asp256----Val); PiMmalton (Phe51----deletion) and PiI (Arg39----Cys). Hum Genet. 1989 Dec;84(1):55–58. doi: 10.1007/BF00210671. [DOI] [PubMed] [Google Scholar]
- Grompe M., Muzny D. M., Caskey C. T. Scanning detection of mutations in human ornithine transcarbamoylase by chemical mismatch cleavage. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5888–5892. doi: 10.1073/pnas.86.15.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grompe M. The rapid detection of unknown mutations in nucleic acids. Nat Genet. 1993 Oct;5(2):111–117. doi: 10.1038/ng1093-111. [DOI] [PubMed] [Google Scholar]
- Haris I. I., Green P. M., Bentley D. R., Giannelli F. Mutation detection by fluorescent chemical cleavage: application to hemophilia B. PCR Methods Appl. 1994 Apr;3(5):268–271. doi: 10.1101/gr.3.5.268. [DOI] [PubMed] [Google Scholar]
- Jackson J., Sim R. B., Whelan A., Feighery C. An IgG autoantibody which inactivates C1-inhibitor. Nature. 1986 Oct 23;323(6090):722–724. doi: 10.1038/323722a0. [DOI] [PubMed] [Google Scholar]
- Janson M., Larsson C., Werelius B., Jones C., Glaser T., Nakamura Y., Jones C. P., Nordenskjöld M. Detailed physical map of human chromosomal region 11q12-13 shows high meiotic recombination rate around the MEN1 locus. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10609–10613. doi: 10.1073/pnas.88.23.10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivisto U. M., Palvimo J. J., Jänne O. A., Kontula K. A single-base substitution in the proximal Sp1 site of the human low density lipoprotein receptor promoter as a cause of heterozygous familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10526–10530. doi: 10.1073/pnas.91.22.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok P. Y., Carlson C., Yager T. D., Ankener W., Nickerson D. A. Comparative analysis of human DNA variations by fluorescence-based sequencing of PCR products. Genomics. 1994 Sep 1;23(1):138–144. doi: 10.1006/geno.1994.1469. [DOI] [PubMed] [Google Scholar]
- La Rocca E., Amoroso S., Brai M., Di Leonardo S., Aricò M. Angioedema ereditario. Indagini genealogiche e considerazioni cliniche sulle due forme genetiche in una casistica di 10 pazienti. G Ital Dermatol Venereol. 1986 May-Jun;121(3):203–208. [PubMed] [Google Scholar]
- Lane D. A., Olds R. J., Conard J., Boisclair M., Bock S. C., Hultin M., Abildgaard U., Ireland H., Thompson E., Sas G. Pleiotropic effects of antithrombin strand 1C substitution mutations. J Clin Invest. 1992 Dec;90(6):2422–2433. doi: 10.1172/JCI116133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashal R. D., Koontz J., Sklar J. Detection of mutations by cleavage of DNA heteroduplexes with bacteriophage resolvases. Nat Genet. 1995 Feb;9(2):177–183. doi: 10.1038/ng0295-177. [DOI] [PubMed] [Google Scholar]
- Millar D. S., Wacey A. I., Ribando J., Melissari E., Laursen B., Woods P., Kakkar V. V., Cooper D. N. Three novel missense mutations in the antithrombin III (AT3) gene causing recurrent venous thrombosis. Hum Genet. 1994 Nov;94(5):509–512. doi: 10.1007/BF00211016. [DOI] [PubMed] [Google Scholar]
- Newburger P. E., Skalnik D. G., Hopkins P. J., Eklund E. A., Curnutte J. T. Mutations in the promoter region of the gene for gp91-phox in X-linked chronic granulomatous disease with decreased expression of cytochrome b558. J Clin Invest. 1994 Sep;94(3):1205–1211. doi: 10.1172/JCI117437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D. J., Carrell R. W. Molecular genetics of human antithrombin deficiency. Hum Mutat. 1996;7(1):7–22. doi: 10.1002/(SICI)1098-1004(1996)7:1<7::AID-HUMU2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Phelps R. S., Chadwick R. B., Conrad M. P., Kronick M. N., Kamb A. Efficient, automatic detection of heterozygous bases during large-scale DNA sequence screening. Biotechniques. 1995 Dec;19(6):984–989. [PubMed] [Google Scholar]
- ROSEN F. S., PENSKY J., DONALDSON V., CHARACHE P. HEREDITARY ANGIONEUROTIC EDEMA: TWO GENETIC VARIANTS. Science. 1965 May 14;148(3672):957–958. doi: 10.1126/science.148.3672.957. [DOI] [PubMed] [Google Scholar]
- Ronchi A., Nicolis S., Santoro C., Ottolenghi S. Increased Sp1 binding mediates erythroid-specific overexpression of a mutated (HPFH) gamma-globulin promoter. Nucleic Acids Res. 1989 Dec 25;17(24):10231–10241. doi: 10.1093/nar/17.24.10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley G., Saad S., Giannelli F., Green P. M. Ultrarapid mutation detection by multiplex, solid-phase chemical cleavage. Genomics. 1995 Dec 10;30(3):574–582. doi: 10.1006/geno.1995.1279. [DOI] [PubMed] [Google Scholar]
- Sakai T., Ohtani N., McGee T. L., Robbins P. D., Dryja T. P. Oncogenic germ-line mutations in Sp1 and ATF sites in the human retinoblastoma gene. Nature. 1991 Sep 5;353(6339):83–86. doi: 10.1038/353083a0. [DOI] [PubMed] [Google Scholar]
- Senapathy P., Shapiro M. B., Harris N. L. Splice junctions, branch point sites, and exons: sequence statistics, identification, and applications to genome project. Methods Enzymol. 1990;183:252–278. doi: 10.1016/0076-6879(90)83018-5. [DOI] [PubMed] [Google Scholar]
- Siddique Z., McPhaden A. R., Fothergill J. E., Whaley K. A point mutation in the C1-inhibitor gene causes type I hereditary angiooedema. Hum Hered. 1993 May-Jun;43(3):155–158. doi: 10.1159/000154171. [DOI] [PubMed] [Google Scholar]
- Siddique Z., McPhaden A. R., Lappin D. F., Whaley K. An RNA splice site mutation in the C1-inhibitor gene causes type I hereditary angio-oedema. Hum Genet. 1991 Dec;88(2):231–232. doi: 10.1007/BF00206079. [DOI] [PubMed] [Google Scholar]
- Siddique Z., McPhaden A. R., McCluskey D., Whaley K. A single base deletion from the C1-inhibitor gene causes type I hereditary angio-oedema. Hum Hered. 1992;42(4):231–234. doi: 10.1159/000154075. [DOI] [PubMed] [Google Scholar]
- Stein P. E., Carrell R. W. What do dysfunctional serpins tell us about molecular mobility and disease? Nat Struct Biol. 1995 Feb;2(2):96–113. doi: 10.1038/nsb0295-96. [DOI] [PubMed] [Google Scholar]
- Stoppa-Lyonnet D., Duponchel C., Meo T., Laurent J., Carter P. E., Arala-Chaves M., Cohen J. H., Dewald G., Goetz J., Hauptmann G. Recombinational biases in the rearranged C1-inhibitor genes of hereditary angioedema patients. Am J Hum Genet. 1991 Nov;49(5):1055–1062. [PMC free article] [PubMed] [Google Scholar]
- Stoppa-Lyonnet D., Tosi M., Laurent J., Sobel A., Lagrue G., Meo T. Altered C1 inhibitor genes in type I hereditary angioedema. N Engl J Med. 1987 Jul 2;317(1):1–6. doi: 10.1056/NEJM198707023170101. [DOI] [PubMed] [Google Scholar]
- Sykes K., Kaufman R. A naturally occurring gamma globin gene mutation enhances SP1 binding activity. Mol Cell Biol. 1990 Jan;10(1):95–102. doi: 10.1128/mcb.10.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verpy E., Biasotto M., Meo T., Tosi M. Efficient detection of point mutations on color-coded strands of target DNA. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1873–1877. doi: 10.1073/pnas.91.5.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verpy E., Couture-Tosi E., Eldering E., Lopez-Trascasa M., Späth P., Meo T., Tosi M. Crucial residues in the carboxy-terminal end of C1 inhibitor revealed by pathogenic mutants impaired in secretion or function. J Clin Invest. 1995 Jan;95(1):350–359. doi: 10.1172/JCI117663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youil R., Kemper B. W., Cotton R. G. Screening for mutations by enzyme mismatch cleavage with T4 endonuclease VII. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):87–91. doi: 10.1073/pnas.92.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedi K., Prada A. E., Davis A. E., 3rd Transcriptional regulation of the C1 inhibitor gene by gamma-interferon. J Biol Chem. 1994 Apr 1;269(13):9669–9674. [PubMed] [Google Scholar]




