Abstract
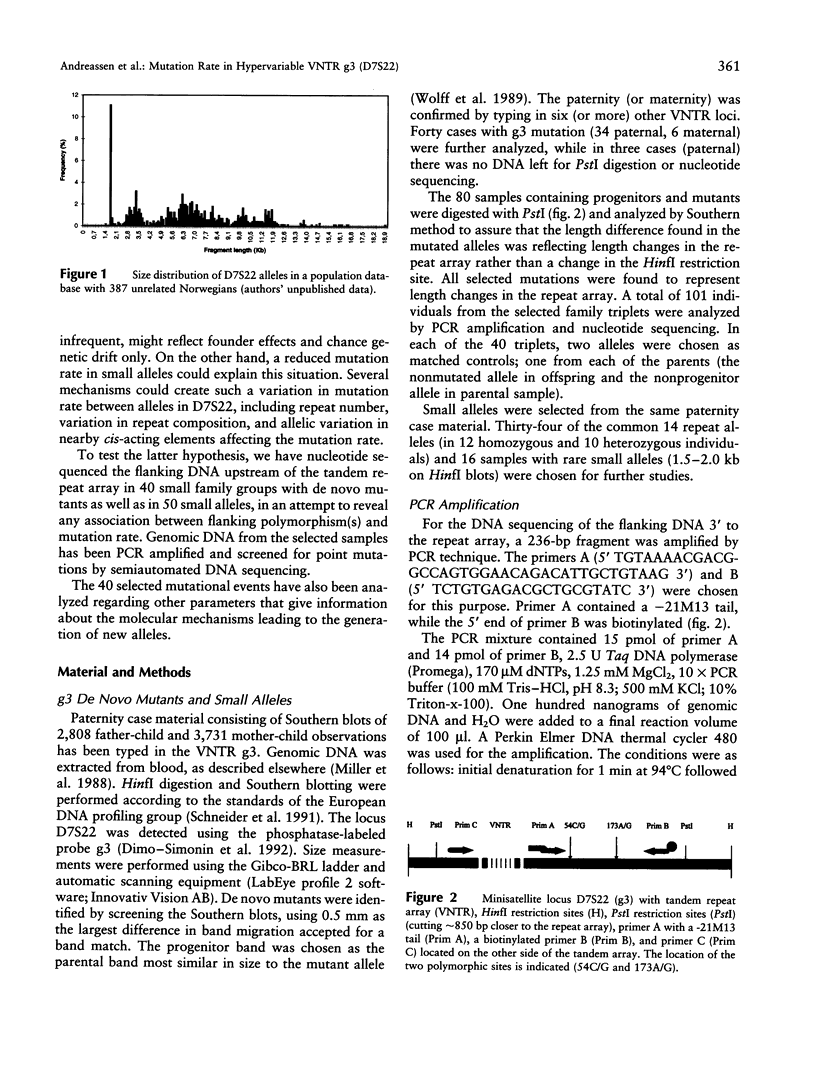
The hypervariable human minisatellite locus D7S22 (g3) is highly polymorphic. The allelic distribution in D7S22 features a size clustering of the alleles and a comparably low allelic diversity among small alleles. This reduced diversity could reflect a situation where some alleles are less likely to mutate than others. Several factors could explain such an effect, including allele size, variation in repeat composition, and allelic differences in nearby cis-acting elements affecting the mutation rate. We have characterized 40 de novo mutations found on Southern blots in a large amount of paternity-testing material. There is a significant excess of paternal mutations, and small size changes are most frequent. Mutation rate is affected by allele length, with highest rates in larger alleles. Alleles of the family groups with D7S22 mutations and 50 small alleles were analyzed by nucleotide sequencing. Two hundred thirty-six base pairs of the immediate flanking region upstream of the repeat array were PCR amplified and screened for point mutations by DNA sequencing of the PCR products. Two base substitution polymorphisms were identified: one C/G transversion and one A/G transition, 54 bp and 173 bp upstream of the repeat array, respectively. There is a significant association between mutation and occurrence of 54C, while association is not obvious between mutation rate and the 173A/G variants. There is a marked association between different flanking haplotypes and allele size, and within the smallest allele-size group, all alleles had the 54G/173A haplotype. Both allele size and allelic state at site 54 remain associated with mutation rate when the other factor is controlled. Possible mechanisms behind the variation in mutation rate in D7S22 are discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armour J. A., Harris P. C., Jeffreys A. J. Allelic diversity at minisatellite MS205 (D16S309): evidence for polarized variability. Hum Mol Genet. 1993 Aug;2(8):1137–1145. doi: 10.1093/hmg/2.8.1137. [DOI] [PubMed] [Google Scholar]
- Baumruker T., Sturm R., Herr W. OBP100 binds remarkably degenerate octamer motifs through specific interactions with flanking sequences. Genes Dev. 1988 Nov;2(11):1400–1413. doi: 10.1101/gad.2.11.1400. [DOI] [PubMed] [Google Scholar]
- Buard J., Vergnaud G. Complex recombination events at the hypermutable minisatellite CEB1 (D2S90). EMBO J. 1994 Jul 1;13(13):3203–3210. doi: 10.1002/j.1460-2075.1994.tb06619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung M. Y., Ranum L. P., Duvick L. A., Servadio A., Zoghbi H. Y., Orr H. T. Evidence for a mechanism predisposing to intergenerational CAG repeat instability in spinocerebellar ataxia type I. Nat Genet. 1993 Nov;5(3):254–258. doi: 10.1038/ng1193-254. [DOI] [PubMed] [Google Scholar]
- Dimo-Simonin N., Brandt-Casadevall C., Gujer H. R. Chemiluminescent DNA probes: evaluation and usefulness in forensic cases. Forensic Sci Int. 1992 Dec;57(2):119–127. doi: 10.1016/0379-0738(92)90004-g. [DOI] [PubMed] [Google Scholar]
- Eichler E. E., Holden J. J., Popovich B. W., Reiss A. L., Snow K., Thibodeau S. N., Richards C. S., Ward P. A., Nelson D. L. Length of uninterrupted CGG repeats determines instability in the FMR1 gene. Nat Genet. 1994 Sep;8(1):88–94. doi: 10.1038/ng0994-88. [DOI] [PubMed] [Google Scholar]
- Falkner F. G., Zachau H. G. Correct transcription of an immunoglobulin kappa gene requires an upstream fragment containing conserved sequence elements. Nature. 1984 Jul 5;310(5972):71–74. doi: 10.1038/310071a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., MacLeod A., Tamaki K., Neil D. L., Monckton D. G. Minisatellite repeat coding as a digital approach to DNA typing. Nature. 1991 Nov 21;354(6350):204–209. doi: 10.1038/354204a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Neumann R., Wilson V. Repeat unit sequence variation in minisatellites: a novel source of DNA polymorphism for studying variation and mutation by single molecule analysis. Cell. 1990 Feb 9;60(3):473–485. doi: 10.1016/0092-8674(90)90598-9. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Royle N. J., Wilson V., Wong Z. Spontaneous mutation rates to new length alleles at tandem-repetitive hypervariable loci in human DNA. Nature. 1988 Mar 17;332(6161):278–281. doi: 10.1038/332278a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Tamaki K., MacLeod A., Monckton D. G., Neil D. L., Armour J. A. Complex gene conversion events in germline mutation at human minisatellites. Nat Genet. 1994 Feb;6(2):136–145. doi: 10.1038/ng0294-136. [DOI] [PubMed] [Google Scholar]
- Leren T. P., Rødningen O. K., Røsby O., Solberg K., Berg K. Screening for point mutations by semi-automated DNA sequencing using sequenase and magnetic beads. Biotechniques. 1993 Apr;14(4):618–623. [PubMed] [Google Scholar]
- Levinson G., Gutman G. A. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol Biol Evol. 1987 May;4(3):203–221. doi: 10.1093/oxfordjournals.molbev.a040442. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monckton D. G., Neumann R., Guram T., Fretwell N., Tamaki K., MacLeod A., Jeffreys A. J. Minisatellite mutation rate variation associated with a flanking DNA sequence polymorphism. Nat Genet. 1994 Oct;8(2):162–170. doi: 10.1038/ng1094-162. [DOI] [PubMed] [Google Scholar]
- Neil D. L., Jeffreys A. J. Digital DNA typing at a second hypervariable locus by minisatellite variant repeat mapping. Hum Mol Genet. 1993 Aug;2(8):1129–1135. doi: 10.1093/hmg/2.8.1129. [DOI] [PubMed] [Google Scholar]
- Olaisen B., Bekkemoen M., Hoff-Olsen P., Gill P. Human VNTR mutation and sex. EXS. 1993;67:63–69. doi: 10.1007/978-3-0348-8583-6_6. [DOI] [PubMed] [Google Scholar]
- Parslow T. G., Blair D. L., Murphy W. J., Granner D. K. Structure of the 5' ends of immunoglobulin genes: a novel conserved sequence. Proc Natl Acad Sci U S A. 1984 May;81(9):2650–2654. doi: 10.1073/pnas.81.9.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau F., Rouillard P., Morel M. L., Khandjian E. W., Morgan K. Prevalence of carriers of premutation-size alleles of the FMRI gene--and implications for the population genetics of the fragile X syndrome. Am J Hum Genet. 1995 Nov;57(5):1006–1018. [PMC free article] [PubMed] [Google Scholar]
- Schneider P. M., Fimmers R., Woodroffe S., Werrett D. J., Bär W., Brinkmann B., Eriksen B., Jones S., Kloosterman A. D., Mevåg B. Report of a European collaborative exercise comparing DNA typing results using a single locus VNTR probe. Forensic Sci Int. 1991 Jan-Feb;49(1):1–15. doi: 10.1016/0379-0738(91)90166-g. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Anwar R., Riley J., Jenner D., Markham A. F., Jeffreys A. J. Highly polymorphic minisatellite sequences: allele frequencies and mutation rates for five locus-specific probes in a Caucasian population. J Forensic Sci Soc. 1990 Jan-Feb;30(1):19–32. doi: 10.1016/s0015-7368(90)73298-7. [DOI] [PubMed] [Google Scholar]
- Vogel F., Rathenberg R. Spontaneous mutation in man. Adv Hum Genet. 1975;5:223–318. doi: 10.1007/978-1-4615-9068-2_4. [DOI] [PubMed] [Google Scholar]
- Wolff R. K., Plaetke R., Jeffreys A. J., White R. Unequal crossingover between homologous chromosomes is not the major mechanism involved in the generation of new alleles at VNTR loci. Genomics. 1989 Aug;5(2):382–384. doi: 10.1016/0888-7543(89)90076-1. [DOI] [PubMed] [Google Scholar]
- Wong Z., Wilson V., Jeffreys A. J., Thein S. L. Cloning a selected fragment from a human DNA 'fingerprint': isolation of an extremely polymorphic minisatellite. Nucleic Acids Res. 1986 Jun 11;14(11):4605–4616. doi: 10.1093/nar/14.11.4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Friedlander P., Lamoureux C., Zannis-Hadjopoulos M., Price G. B. cDNA clones contain autonomous replication activity. Biochim Biophys Acta. 1993 Sep 23;1174(3):241–257. doi: 10.1016/0167-4781(93)90193-h. [DOI] [PubMed] [Google Scholar]





