Abstract
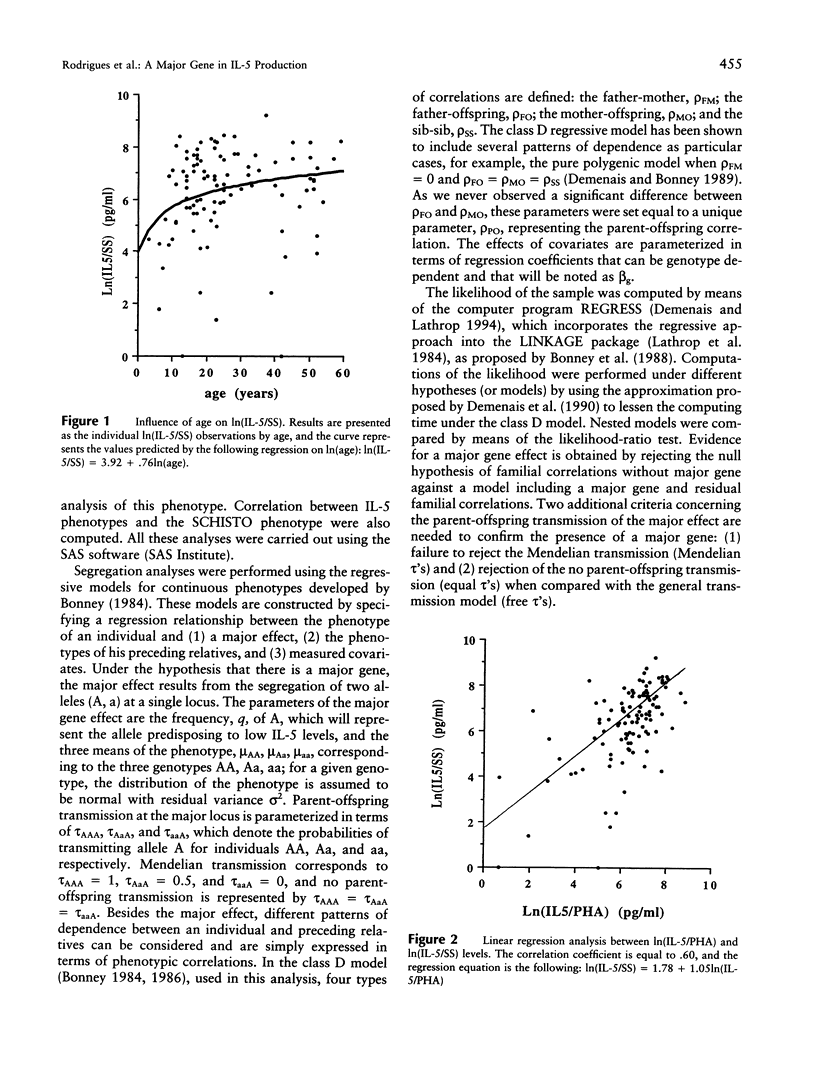
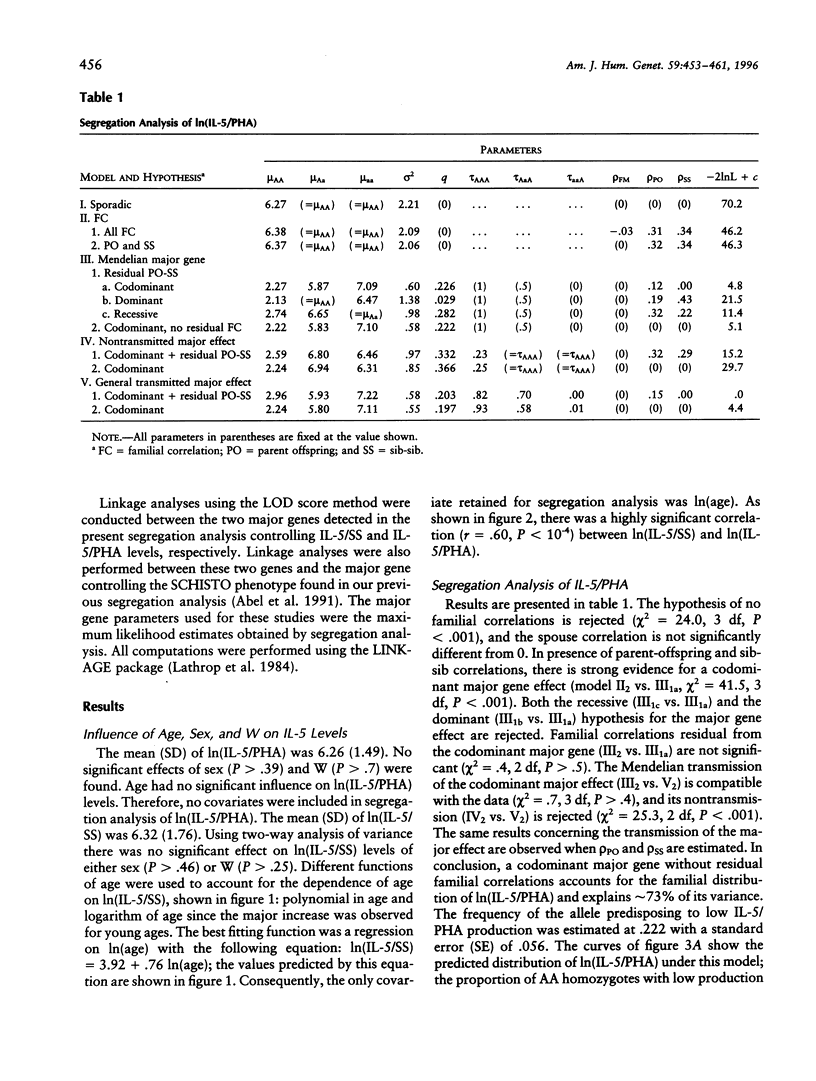
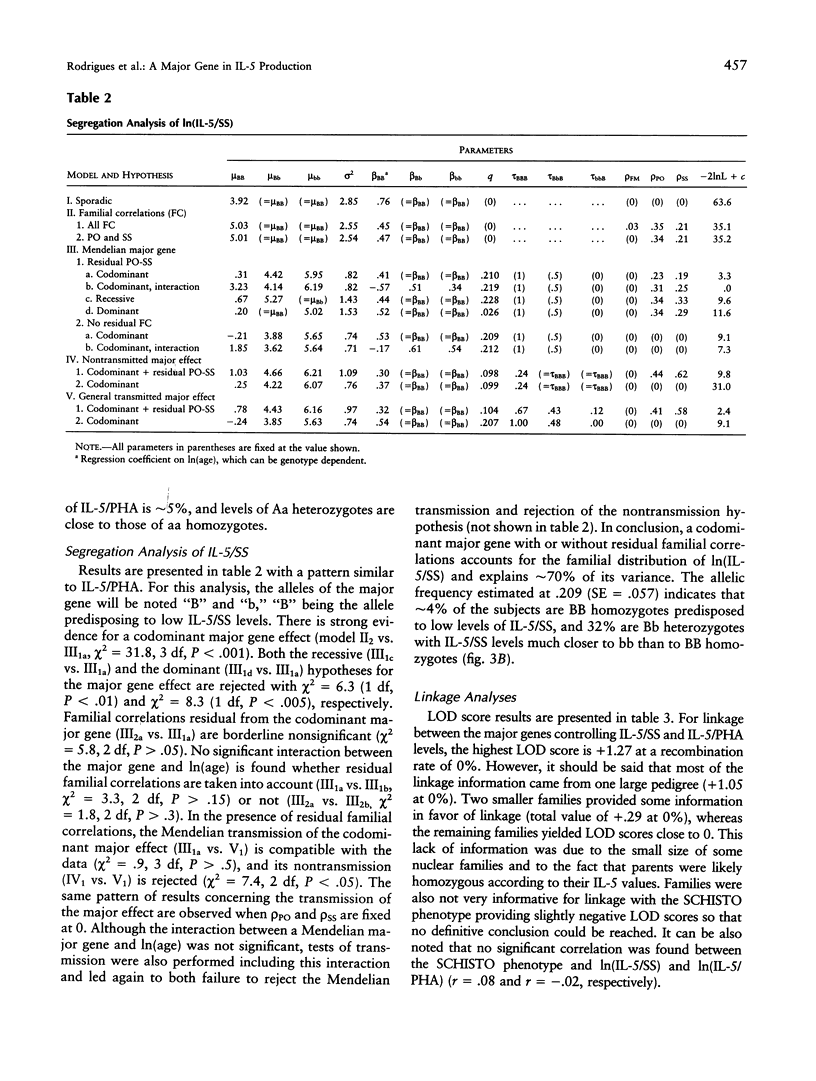
The interleukine-5 (IL-5) is a hormone of the immune system that is the main regulator of eosinopoiesis, eosinophil maturation and activation, and immunoglobulin A production. Thus, IL-5 contributes in several ways to human immune defenses against various pathogens, including helminths and infectious agents of the digestive and respiratory tracts. On the other hand, the increase in eosinophil number and the activation of these cells, which both have been related to elevated IL-5 production, are the cause of severe pathological disorders, as in asthma or hypereosinophilic syndromes. Although the immunological pathways leading to IL-5 synthesis have been identified, the reasons for the large variability observed in IL-5 production among subjects exposed to comparable antigenic stimulation are unknown. To investigate the role of genetic factors in this variability, we conducted a segregation analysis in a Brazilian population infected by the helminth parasite Schistosoma mansoni. The analysis was performed on IL-5 levels produced by blood mononuclear cells of these subjects after in vitro restimulation with either parasite extracts (IL-5/schistosomula sonicates [SS] phenotype) or a T-lymphocyte mitogen (IL-5/phytohemagglutin [PHA]). The results provide clear evidence for the segregation of a codominant major gene controlling IL-5/SS and IL-5/PHA production and accounting for 70% and 73% of the phenotypic variance, respectively; the frequency of the allele predisposing to low IL-5 production was approximately .22 for both phenotypes. No significant relationship was found between these genes and the gene controlling infection intensities by S. mansoni detected in a previous study. Linkage studies are ongoing to locate those genes that would help to characterize the genetic factors involved in pathological conditions such as severe helminth infections and allergic diseases.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel L., Demenais F., Prata A., Souza A. E., Dessein A. Evidence for the segregation of a major gene in human susceptibility/resistance to infection by Schistosoma mansoni. Am J Hum Genet. 1991 May;48(5):959–970. [PMC free article] [PubMed] [Google Scholar]
- Bonney G. E., Lathrop G. M., Lalouel J. M. Combined linkage and segregation analysis using regressive models. Am J Hum Genet. 1988 Jul;43(1):29–37. [PMC free article] [PubMed] [Google Scholar]
- Bonney G. E. On the statistical determination of major gene mechanisms in continuous human traits: regressive models. Am J Med Genet. 1984 Aug;18(4):731–749. doi: 10.1002/ajmg.1320180420. [DOI] [PubMed] [Google Scholar]
- Broide D. H., Paine M. M., Firestein G. S. Eosinophils express interleukin 5 and granulocyte macrophage-colony-stimulating factor mRNA at sites of allergic inflammation in asthmatics. J Clin Invest. 1992 Oct;90(4):1414–1424. doi: 10.1172/JCI116008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth A. E., Sturrock R. F., Houba V., Mahmoud A. A., Sher A., Rees P. H. Eosinophils as mediators of antibody-dependent damage to schistosomula. Nature. 1975 Aug 28;256(5520):727–729. doi: 10.1038/256727a0. [DOI] [PubMed] [Google Scholar]
- Butterworth A. E., Sturrock R. F., Houba V., Rees P. H. Antibody-dependent cell-mediated damage to schistosomula in vitro. Nature. 1974 Dec 6;252(5483):503–505. doi: 10.1038/252503a0. [DOI] [PubMed] [Google Scholar]
- Butterworth A. E. The eosinophil and its role in immunity to helminth infection. Curr Top Microbiol Immunol. 1977;77:127–168. doi: 10.1007/978-3-642-66740-4_5. [DOI] [PubMed] [Google Scholar]
- Campbell H. D., Tucker W. Q., Hort Y., Martinson M. E., Mayo G., Clutterbuck E. J., Sanderson C. J., Young I. G. Molecular cloning, nucleotide sequence, and expression of the gene encoding human eosinophil differentiation factor (interleukin 5). Proc Natl Acad Sci U S A. 1987 Oct;84(19):6629–6633. doi: 10.1073/pnas.84.19.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chand N., Harrison J. E., Rooney S., Pillar J., Jakubicki R., Nolan K., Diamantis W., Sofia R. D. Anti-IL-5 monoclonal antibody inhibits allergic late phase bronchial eosinophilia in guinea pigs: a therapeutic approach. Eur J Pharmacol. 1992 Jan 28;211(1):121–123. doi: 10.1016/0014-2999(92)90273-7. [DOI] [PubMed] [Google Scholar]
- Childers N. K., Bruce M. G., McGhee J. R. Molecular mechanisms of immunoglobulin A defense. Annu Rev Microbiol. 1989;43:503–536. doi: 10.1146/annurev.mi.43.100189.002443. [DOI] [PubMed] [Google Scholar]
- Clutterbuck E. J., Sanderson C. J. Human eosinophil hematopoiesis studied in vitro by means of murine eosinophil differentiation factor (IL5): production of functionally active eosinophils from normal human bone marrow. Blood. 1988 Mar;71(3):646–651. [PubMed] [Google Scholar]
- Coffman R. L., Seymour B. W., Hudak S., Jackson J., Rennick D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science. 1989 Jul 21;245(4915):308–310. doi: 10.1126/science.2787531. [DOI] [PubMed] [Google Scholar]
- Couissinier-Paris P., Dessein A. J. Schistosoma-specific helper T cell clones from subjects resistant to infection by Schistosoma mansoni are Th0/2. Eur J Immunol. 1995 Aug;25(8):2295–2302. doi: 10.1002/eji.1830250827. [DOI] [PubMed] [Google Scholar]
- Coëffier E., Joseph D., Vargaftig B. B. Activation of guinea pig eosinophils by human recombinant IL-5. Selective priming to platelet-activating factor-acether and interference of its antagonists. J Immunol. 1991 Oct 15;147(8):2595–2602. [PubMed] [Google Scholar]
- Demenais F. M., Bonney G. E. Equivalence of the mixed and regressive models for genetic analysis. I. Continuous traits. Genet Epidemiol. 1989;6(5):597–617. doi: 10.1002/gepi.1370060505. [DOI] [PubMed] [Google Scholar]
- Demenais F. M., Murigande C., Bonney G. E. Search for faster methods of fitting the regressive models to quantitative traits. Genet Epidemiol. 1990;7(5):319–334. doi: 10.1002/gepi.1370070503. [DOI] [PubMed] [Google Scholar]
- Demeure C. E., Rihet P., Abel L., Ouattara M., Bourgois A., Dessein A. J. Resistance to Schistosoma mansoni in humans: influence of the IgE/IgG4 balance and IgG2 in immunity to reinfection after chemotherapy. J Infect Dis. 1993 Oct;168(4):1000–1008. doi: 10.1093/infdis/168.4.1000. [DOI] [PubMed] [Google Scholar]
- Dent L. A., Strath M., Mellor A. L., Sanderson C. J. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med. 1990 Nov 1;172(5):1425–1431. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desreumaux P., Janin A., Dubucquoi S., Copin M. C., Torpier G., Capron A., Capron M., Prin L. Synthesis of interleukin-5 by activated eosinophils in patients with eosinophilic heart diseases. Blood. 1993 Sep 1;82(5):1553–1560. [PubMed] [Google Scholar]
- Dessein A. J., Lenzi H. L., Bina J. C., Carvalho E. M., Weiser W. Y., Andrade Z. A., David J. R. Modulation of eosinophil cytotoxicity by blood mononuclear cells from healthy subjects and patients with chronic schistosomiasis mansoni. Cell Immunol. 1984 Apr 15;85(1):100–113. doi: 10.1016/0008-8749(84)90282-x. [DOI] [PubMed] [Google Scholar]
- Dessein A. J., Lenzi H. L., David J. R. Modulation of the cytotoxicity of human blood eosinophils by factors secreted by monocytes and T lymphocytes. Monogr Allergy. 1983;18:45–51. [PubMed] [Google Scholar]
- Dessein A. J., Vadas M. A., Nicola N. A., Metcalf D., David J. R. Enhancement of human blood eosinophil cytotoxicity by semi-purified eosinophil colony-stimulating factor(s). J Exp Med. 1982 Jul 1;156(1):90–103. doi: 10.1084/jem.156.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleich G. J., Frigas E., Loegering D. A., Wassom D. L., Steinmuller D. Cytotoxic properties of the eosinophil major basic protein. J Immunol. 1979 Dec;123(6):2925–2927. [PubMed] [Google Scholar]
- Hamid Q., Azzawi M., Ying S., Moqbel R., Wardlaw A. J., Corrigan C. J., Bradley B., Durham S. R., Collins J. V., Jeffery P. K. Expression of mRNA for interleukin-5 in mucosal bronchial biopsies from asthma. J Clin Invest. 1991 May;87(5):1541–1546. doi: 10.1172/JCI115166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harriman G. R., Kunimoto D. Y., Elliott J. F., Paetkau V., Strober W. The role of IL-5 in IgA B cell differentiation. J Immunol. 1988 May 1;140(9):3033–3039. [PubMed] [Google Scholar]
- Korenaga M., Hitoshi Y., Yamaguchi N., Sato Y., Takatsu K., Tada I. The role of interleukin-5 in protective immunity to Strongyloides venezuelensis infection in mice. Immunology. 1991 Apr;72(4):502–507. [PMC free article] [PubMed] [Google Scholar]
- Krishnaswamy G., Liu M. C., Su S. N., Kumai M., Xiao H. Q., Marsh D. G., Huang S. K. Analysis of cytokine transcripts in the bronchoalveolar lavage cells of patients with asthma. Am J Respir Cell Mol Biol. 1993 Sep;9(3):279–286. doi: 10.1165/ajrcmb/9.3.279. [DOI] [PubMed] [Google Scholar]
- Lange A. M., Yutanawiboonchai W., Scott P., Abraham D. IL-4- and IL-5-dependent protective immunity to Onchocerca volvulus infective larvae in BALB/cBYJ mice. J Immunol. 1994 Jul 1;153(1):205–211. [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A. F., Sanderson C. J., Gamble J. R., Campbell H. D., Young I. G., Vadas M. A. Recombinant human interleukin 5 is a selective activator of human eosinophil function. J Exp Med. 1988 Jan 1;167(1):219–224. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh D. G., Neely J. D., Breazeale D. R., Ghosh B., Freidhoff L. R., Ehrlich-Kautzky E., Schou C., Krishnaswamy G., Beaty T. H. Linkage analysis of IL4 and other chromosome 5q31.1 markers and total serum immunoglobulin E concentrations. Science. 1994 May 20;264(5162):1152–1156. doi: 10.1126/science.8178175. [DOI] [PubMed] [Google Scholar]
- Mauser P. J., Pitman A., Witt A., Fernandez X., Zurcher J., Kung T., Jones H., Watnick A. S., Egan R. W., Kreutner W. Inhibitory effect of the TRFK-5 anti-IL-5 antibody in a guinea pig model of asthma. Am Rev Respir Dis. 1993 Dec;148(6 Pt 1):1623–1627. doi: 10.1164/ajrccm/148.6_Pt_1.1623. [DOI] [PubMed] [Google Scholar]
- Meyers D. A., Postma D. S., Panhuysen C. I., Xu J., Amelung P. J., Levitt R. C., Bleecker E. R. Evidence for a locus regulating total serum IgE levels mapping to chromosome 5. Genomics. 1994 Sep 15;23(2):464–470. doi: 10.1006/geno.1994.1524. [DOI] [PubMed] [Google Scholar]
- Murray P. D., McKenzie D. T., Swain S. L., Kagnoff M. F. Interleukin 5 and interleukin 4 produced by Peyer's patch T cells selectively enhance immunoglobulin A expression. J Immunol. 1987 Oct 15;139(8):2669–2674. [PubMed] [Google Scholar]
- Nakajima H., Iwamoto I., Tomoe S., Matsumura R., Tomioka H., Takatsu K., Yoshida S. CD4+ T-lymphocytes and interleukin-5 mediate antigen-induced eosinophil infiltration into the mouse trachea. Am Rev Respir Dis. 1992 Aug;146(2):374–377. doi: 10.1164/ajrccm/146.2.374. [DOI] [PubMed] [Google Scholar]
- Olsson I., Venge P. The role of the eosinophil granulocyte in the inflammatory reaction. Allergy. 1979 Dec;34(6):353–367. doi: 10.1111/j.1398-9995.1979.tb02005.x. [DOI] [PubMed] [Google Scholar]
- Postma D. S., Bleecker E. R., Amelung P. J., Holroyd K. J., Xu J., Panhuysen C. I., Meyers D. A., Levitt R. C. Genetic susceptibility to asthma--bronchial hyperresponsiveness coinherited with a major gene for atopy. N Engl J Med. 1995 Oct 5;333(14):894–900. doi: 10.1056/NEJM199510053331402. [DOI] [PubMed] [Google Scholar]
- Roberts M., Butterworth A. E., Kimani G., Kamau T., Fulford A. J., Dunne D. W., Ouma J. H., Sturrock R. F. Immunity after treatment of human schistosomiasis: association between cellular responses and resistance to reinfection. Infect Immun. 1993 Dec;61(12):4984–4993. doi: 10.1128/iai.61.12.4984-4993.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D. S., Hamid Q., Ying S., Tsicopoulos A., Barkans J., Bentley A. M., Corrigan C., Durham S. R., Kay A. B. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992 Jan 30;326(5):298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- Sanderson C. J., Warren D. J., Strath M. Identification of a lymphokine that stimulates eosinophil differentiation in vitro. Its relationship to interleukin 3, and functional properties of eosinophils produced in cultures. J Exp Med. 1985 Jul 1;162(1):60–74. doi: 10.1084/jem.162.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki O., Sugaya H., Ishida K., Yoshimura K. Ablation of eosinophils with anti-IL-5 antibody enhances the survival of intracranial worms of Angiostrongylus cantonensis in the mouse. Parasite Immunol. 1993 Jun;15(6):349–354. doi: 10.1111/j.1365-3024.1993.tb00619.x. [DOI] [PubMed] [Google Scholar]
- Sehmi R., Wardlaw A. J., Cromwell O., Kurihara K., Waltmann P., Kay A. B. Interleukin-5 selectively enhances the chemotactic response of eosinophils obtained from normal but not eosinophilic subjects. Blood. 1992 Jun 1;79(11):2952–2959. [PubMed] [Google Scholar]
- Sonoda E., Matsumoto R., Hitoshi Y., Ishii T., Sugimoto M., Araki S., Tominaga A., Yamaguchi N., Takatsu K. Transforming growth factor beta induces IgA production and acts additively with interleukin 5 for IgA production. J Exp Med. 1989 Oct 1;170(4):1415–1420. doi: 10.1084/jem.170.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spry C. J. Alterations in blood eosinophil morphology, binding capacity for complexed IgG and kinetics in patients with tropical (filarial) eosinophilia. Parasite Immunol. 1981 Spring;3(1):1–11. doi: 10.1111/j.1365-3024.1981.tb00380.x. [DOI] [PubMed] [Google Scholar]
- Spry C. J., Tai P. C. Studies on blood eosinophils. II. Patients with Löffler's cardiomyopathy. Clin Exp Immunol. 1976 Jun;24(3):423–434. [PMC free article] [PubMed] [Google Scholar]
- Tominaga A., Takaki S., Koyama N., Katoh S., Matsumoto R., Migita M., Hitoshi Y., Hosoya Y., Yamauchi S., Kanai Y. Transgenic mice expressing a B cell growth and differentiation factor gene (interleukin 5) develop eosinophilia and autoantibody production. J Exp Med. 1991 Feb 1;173(2):429–437. doi: 10.1084/jem.173.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oosterhout A. J., Ladenius A. R., Savelkoul H. F., Van Ark I., Delsman K. C., Nijkamp F. P. Effect of anti-IL-5 and IL-5 on airway hyperreactivity and eosinophils in guinea pigs. Am Rev Respir Dis. 1993 Mar;147(3):548–552. doi: 10.1164/ajrccm/147.3.548. [DOI] [PubMed] [Google Scholar]
- Voirol M. W., Welsh R. A., Genet E. F. Esophagitis cystica. Am J Gastroenterol. 1973 May;59(5):446–453. [PubMed] [Google Scholar]
- Wang J. M., Rambaldi A., Biondi A., Chen Z. G., Sanderson C. J., Mantovani A. Recombinant human interleukin 5 is a selective eosinophil chemoattractant. Eur J Immunol. 1989 Apr;19(4):701–705. doi: 10.1002/eji.1830190420. [DOI] [PubMed] [Google Scholar]
- Wassom D. L., Loegering D. A., Solley G. O., Moore S. B., Schooley R. T., Fauci A. S., Gleich G. J. Elevated serum levels of the eosinophil granule major basic protein in patients with eosinophilia. J Clin Invest. 1981 Mar;67(3):651–661. doi: 10.1172/JCI110080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Hayashi Y., Sugama Y., Miura Y., Kasahara T., Kitamura S., Torisu M., Mita S., Tominaga A., Takatsu K. Highly purified murine interleukin 5 (IL-5) stimulates eosinophil function and prolongs in vitro survival. IL-5 as an eosinophil chemotactic factor. J Exp Med. 1988 May 1;167(5):1737–1742. doi: 10.1084/jem.167.5.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Suda T., Suda J., Eguchi M., Miura Y., Harada N., Tominaga A., Takatsu K. Purified interleukin 5 supports the terminal differentiation and proliferation of murine eosinophilic precursors. J Exp Med. 1988 Jan 1;167(1):43–56. doi: 10.1084/jem.167.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen B. H., Martinson M. E., Webb G. C., Young I. G. Molecular organization of the cytokine gene cluster, involving the human IL-3, IL-4, IL-5, and GM-CSF genes, on human chromosome 5. Blood. 1989 Apr;73(5):1142–1148. [PubMed] [Google Scholar]



