Abstract
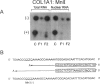
Nonsense and frameshift mutations, which predict premature termination of translation, often cause a dramatic reduction in the amount of transcript from the mutant allele (nonsense-mediated mRNA decay). In some genes, these mutations also influence RNA splicing and induce skipping of the exon that contains the nonsense codon. To begin to dissect how premature termination alters the metabolism of RNA from the COL1A1 gene, we studied nonsense and frameshift mutations distributed over exons 11-49 of the gene. These mutations were originally identified in 10 unrelated families with osteogenesis imperfecta (OI) type 1. We observed marked reduction in steady-state amounts of mRNA from the mutant allele in both total cellular and nuclear RNA extracts of cells from affected individuals, suggesting that nonsense-mediated decay of COL1A1 RNA is a nuclear phenomenon. Position of the mutation within the gene did not influence this observation. None of the mutations induced skipping of either the exon containing the mutation or, for the frameshifts, the downstream exons with the new termination sites. Our data suggest that nonsense and frameshift mutations throughout most of the COL1A1 gene result in a null allele, which is associated with the predictable mild clinical phenotype, OI type 1.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barsh G. S., David K. E., Byers P. H. Type I osteogenesis imperfecta: a nonfunctional allele for pro alpha 1 (I) chains of type I procollagen. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3838–3842. doi: 10.1073/pnas.79.12.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baserga S. J., Benz E. J., Jr Nonsense mutations in the human beta-globin gene affect mRNA metabolism. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2056–2060. doi: 10.1073/pnas.85.7.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgrader P., Cheng J., Zhou X., Stephenson L. S., Maquat L. E. Mammalian nonsense codons can be cis effectors of nuclear mRNA half-life. Mol Cell Biol. 1994 Dec;14(12):8219–8228. doi: 10.1128/mcb.14.12.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgrader P., Maquat L. E. Nonsense but not missense mutations can decrease the abundance of nuclear mRNA for the mouse major urinary protein, while both types of mutations can facilitate exon skipping. Mol Cell Biol. 1994 Sep;14(9):6326–6336. doi: 10.1128/mcb.14.9.6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J., Fogel-Petrovic M., Maquat L. E. Translation to near the distal end of the penultimate exon is required for normal levels of spliced triosephosphate isomerase mRNA. Mol Cell Biol. 1990 Oct;10(10):5215–5225. doi: 10.1128/mcb.10.10.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- D'Alessio M., Bernard M., Pretorius P. J., de Wet W., Ramirez F., Pretorious P. J. Complete nucleotide sequence of the region encompassing the first twenty-five exons of the human pro alpha 1(I) collagen gene (COL1A1) Gene. 1988 Jul 15;67(1):105–115. doi: 10.1016/0378-1119(88)90013-3. [DOI] [PubMed] [Google Scholar]
- Daar I. O., Maquat L. E. Premature translation termination mediates triosephosphate isomerase mRNA degradation. Mol Cell Biol. 1988 Feb;8(2):802–813. doi: 10.1128/mcb.8.2.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Levinson B., Whitney S., Vulpe C., Packman S., Gitschier J. Diverse mutations in patients with Menkes disease often lead to exon skipping. Am J Hum Genet. 1994 Nov;55(5):883–889. [PMC free article] [PubMed] [Google Scholar]
- Dietz H. C., Valle D., Francomano C. A., Kendzior R. J., Jr, Pyeritz R. E., Cutting G. R. The skipping of constitutive exons in vivo induced by nonsense mutations. Science. 1993 Jan 29;259(5095):680–683. doi: 10.1126/science.8430317. [DOI] [PubMed] [Google Scholar]
- Eldadah Z. A., Grifo J. A., Dietz H. C. Marfan syndrome as a paradigm for transcript-targeted preimplantation diagnosis of heterozygous mutations. Nat Med. 1995 Aug;1(8):798–803. doi: 10.1038/nm0895-798. [DOI] [PubMed] [Google Scholar]
- Fisher C. W., Fisher C. R., Chuang J. L., Lau K. S., Chuang D. T., Cox R. P. Occurrence of a 2-bp (AT) deletion allele and a nonsense (G-to-T) mutant allele at the E2 (DBT) locus of six patients with maple syrup urine disease: multiple-exon skipping as a secondary effect of the mutations. Am J Hum Genet. 1993 Feb;52(2):414–424. [PMC free article] [PubMed] [Google Scholar]
- Genovese C., Rowe D. Analysis of cytoplasmic and nuclear messenger RNA in fibroblasts from patients with type I osteogenesis imperfecta. Methods Enzymol. 1987;145:223–235. doi: 10.1016/0076-6879(87)45012-x. [DOI] [PubMed] [Google Scholar]
- Gibson R. A., Hajianpour A., Murer-Orlando M., Buchwald M., Mathew C. G. A nonsense mutation and exon skipping in the Fanconi anaemia group C gene. Hum Mol Genet. 1993 Jun;2(6):797–799. doi: 10.1093/hmg/2.6.797. [DOI] [PubMed] [Google Scholar]
- Leeds P., Wood J. M., Lee B. S., Culbertson M. R. Gene products that promote mRNA turnover in Saccharomyces cerevisiae. Mol Cell Biol. 1992 May;12(5):2165–2177. doi: 10.1128/mcb.12.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. K., Sigmund C. D., Gross K. W., Maquat L. E. Nonsense codons in human beta-globin mRNA result in the production of mRNA degradation products. Mol Cell Biol. 1992 Mar;12(3):1149–1161. doi: 10.1128/mcb.12.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losson R., Lacroute F. Interference of nonsense mutations with eukaryotic messenger RNA stability. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5134–5137. doi: 10.1073/pnas.76.10.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus T., Baserga S. J., Stolle C., Takeshita K., Benz E. J., Jr Metabolism of non-translatable globin mRNAs arising from premature translation termination codons. Ann N Y Acad Sci. 1990;612:55–66. doi: 10.1111/j.1749-6632.1990.tb24290.x. [DOI] [PubMed] [Google Scholar]
- Mashima Y., Murakami A., Weleber R. G., Kennaway N. G., Clarke L., Shiono T., Inana G. Nonsense-codon mutations of the ornithine aminotransferase gene with decreased levels of mutant mRNA in gyrate atrophy. Am J Hum Genet. 1992 Jul;51(1):81–91. [PMC free article] [PubMed] [Google Scholar]
- Ming Y. Z., Di X., Gomez-Sanchez E. P., Gomez-Sanchez C. E. Improved downward capillary transfer for blotting of DNA and RNA. Biotechniques. 1994 Jan;16(1):58–59. [PubMed] [Google Scholar]
- Orita M., Iwahana H., Kanazawa H., Hayashi K., Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poduslo S. E., Dean M., Kolch U., O'Brien S. J. Detecting high-resolution polymorphisms in human coding loci by combining PCR and single-strand conformation polymorphism (SSCP) analysis. Am J Hum Genet. 1991 Jul;49(1):106–111. [PMC free article] [PubMed] [Google Scholar]
- Pulak R., Anderson P. mRNA surveillance by the Caenorhabditis elegans smg genes. Genes Dev. 1993 Oct;7(10):1885–1897. doi: 10.1101/gad.7.10.1885. [DOI] [PubMed] [Google Scholar]
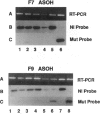
- Redford-Badwal D. A., Stover M. L., Valli M., McKinstry M. B., Rowe D. W. Nuclear retention of COL1A1 messenger RNA identifies null alleles causing mild osteogenesis imperfecta. J Clin Invest. 1996 Feb 15;97(4):1035–1040. doi: 10.1172/JCI118495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe D. W., Shapiro J. R., Poirier M., Schlesinger S. Diminished type I collagen synthesis and reduced alpha 1(I) collagen messenger RNA in cultured fibroblasts from patients with dominantly inherited (type I) osteogenesis imperfecta. J Clin Invest. 1985 Aug;76(2):604–611. doi: 10.1172/JCI112012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillence D. O., Senn A., Danks D. M. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979 Apr;16(2):101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
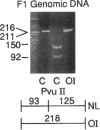
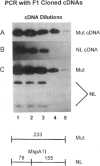
- Stover M. L., Primorac D., Liu S. C., McKinstry M. B., Rowe D. W. Defective splicing of mRNA from one COL1A1 allele of type I collagen in nondeforming (type I) osteogenesis imperfecta. J Clin Invest. 1993 Oct;92(4):1994–2002. doi: 10.1172/JCI116794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes B., Francis M. J., Smith R. Altered relation of two collagen types in osteogenesis imperfecta. N Engl J Med. 1977 May 26;296(21):1200–1203. doi: 10.1056/NEJM197705262962104. [DOI] [PubMed] [Google Scholar]
- Urlaub G., Mitchell P. J., Ciudad C. J., Chasin L. A. Nonsense mutations in the dihydrofolate reductase gene affect RNA processing. Mol Cell Biol. 1989 Jul;9(7):2868–2880. doi: 10.1128/mcb.9.7.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenstrup R. J., Willing M. C., Starman B. J., Byers P. H. Distinct biochemical phenotypes predict clinical severity in nonlethal variants of osteogenesis imperfecta. Am J Hum Genet. 1990 May;46(5):975–982. [PMC free article] [PubMed] [Google Scholar]
- Westerhausen A., Constantinou C. D., Pack M., Peng M. Z., Hanning C., Olsen A. S., Prockop D. J. Completion of the last half of the structure of the human gene for the Pro alpha 1 (I) chain of type I procollagen (COL1A1). Matrix. 1991 Dec;11(6):375–379. doi: 10.1016/s0934-8832(11)80191-5. [DOI] [PubMed] [Google Scholar]
- Willing M. C., Cohn D. H., Byers P. H. Frameshift mutation near the 3' end of the COL1A1 gene of type I collagen predicts an elongated Pro alpha 1(I) chain and results in osteogenesis imperfecta type I. J Clin Invest. 1990 Jan;85(1):282–290. doi: 10.1172/JCI114424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willing M. C., Deschenes S. P., Scott D. A., Byers P. H., Slayton R. L., Pitts S. H., Arikat H., Roberts E. J. Osteogenesis imperfecta type I: molecular heterogeneity for COL1A1 null alleles of type I collagen. Am J Hum Genet. 1994 Oct;55(4):638–647. [PMC free article] [PubMed] [Google Scholar]
- Willing M. C., Pruchno C. J., Atkinson M., Byers P. H. Osteogenesis imperfecta type I is commonly due to a COL1A1 null allele of type I collagen. Am J Hum Genet. 1992 Sep;51(3):508–515. [PMC free article] [PubMed] [Google Scholar]
- Zhang Z. X., Wakamatsu N., Mules E. H., Thomas G. H., Gravel R. A. Impact of premature stop codons on mRNA levels in infantile Sandhoff disease. Hum Mol Genet. 1994 Jan;3(1):139–145. doi: 10.1093/hmg/3.1.139. [DOI] [PubMed] [Google Scholar]