Abstract
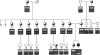
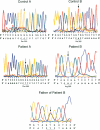
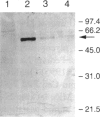
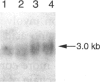
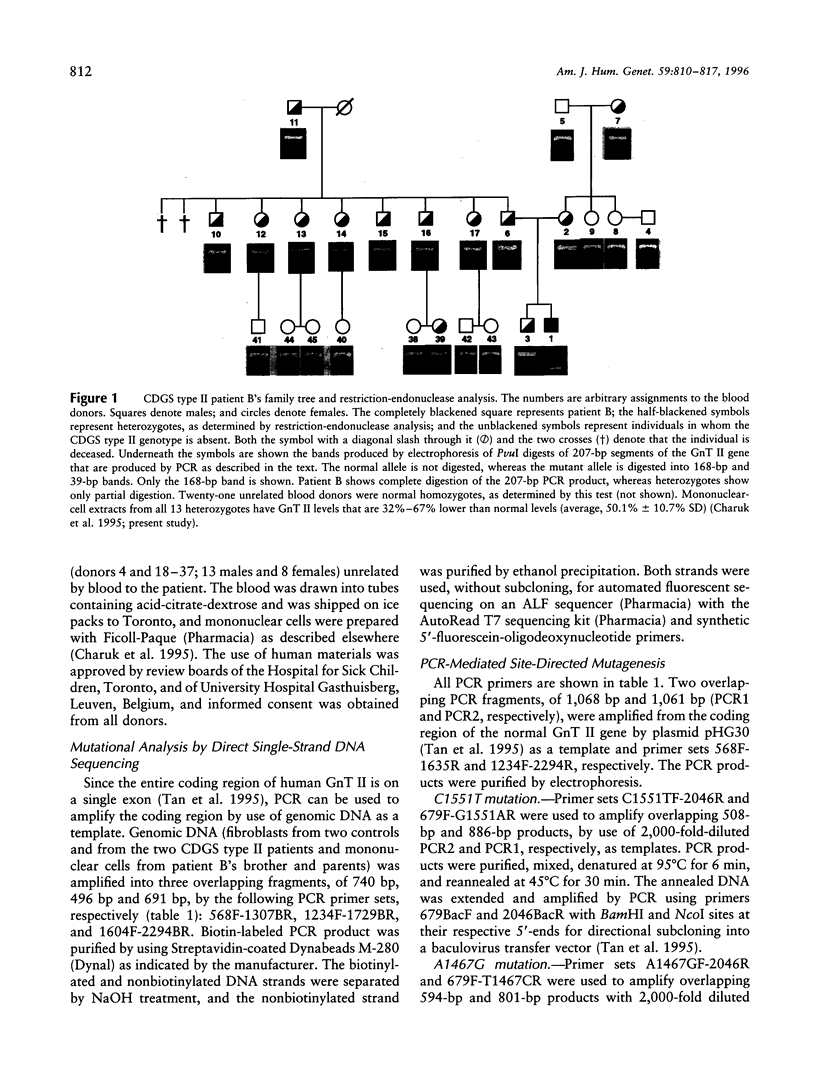
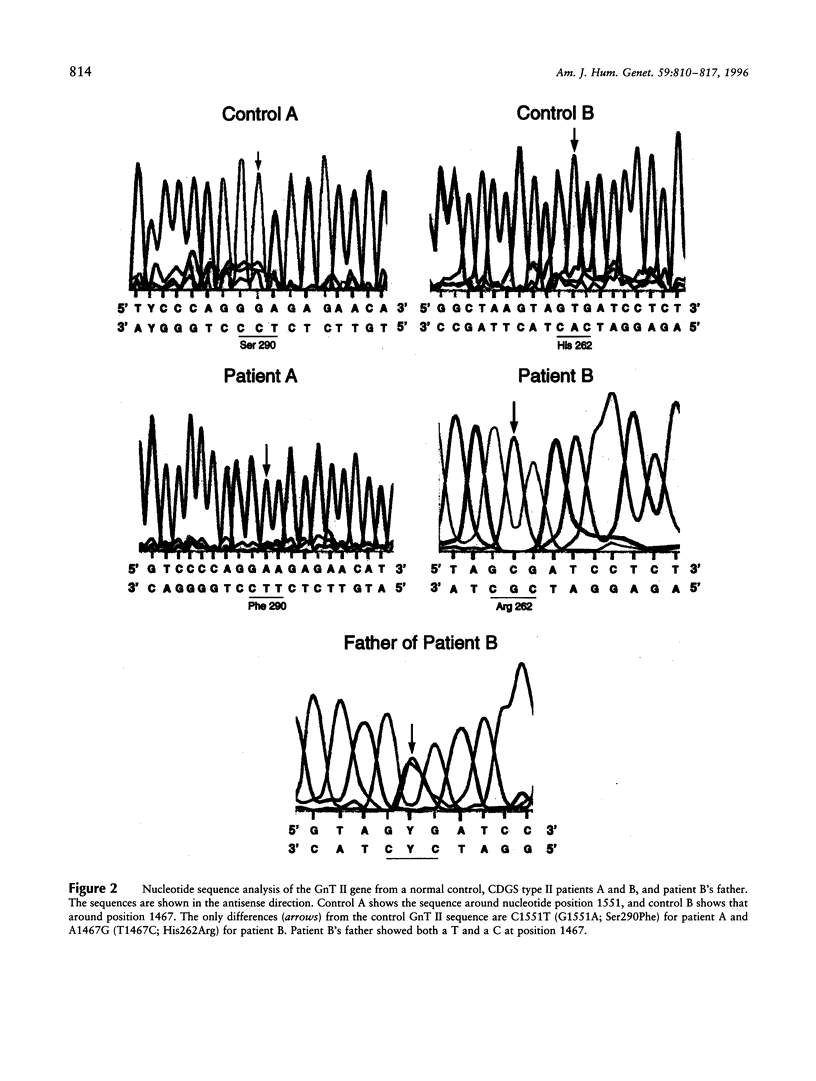
Carbohydrate-deficient glycoprotein syndrome (CDGS) type II is a multisystemic congenital disease with severe involvement of the nervous system. Two unrelated CDGS type II patients are shown to have point mutations (one patient having Ser-->Phe and the other having His-->Arg) in the catalytic domain of the gene MGAT2, encoding UDP-GlcNAc:alpha-6-D-mannoside beta-1,2-N- acetylglucosaminyltransferase II (GnT II), an enzyme essential for biosynthesis of complex Asn-linked glycans. Both mutations caused both decreased expression of enzyme protein in a baculovirus/insect cell system and inactivation of enzyme activity. Restriction-endonuclease analysis of DNA from 23 blood relatives of one of these patients showed that 13 donors were heterozygotes; the other relatives and 21 unrelated donors were normal homozygotes. All heterozygotes showed a significant reduction (33%-68%) in mononuclear-cell GnT II activity. The data indicate that CDGS type II is an autosomal recessive disease and that complex Asn-linked glycans are essential for normal neurological development.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Charuk J. H., Tan J., Bernardini M., Haddad S., Reithmeier R. A., Jaeken J., Schachter H. Carbohydrate-deficient glycoprotein syndrome type II. An autosomal recessive N-acetylglucosaminyltransferase II deficiency different from typical hereditary erythroblastic multinuclearity, with a positive acidified-serum lysis test (HEMPAS). Eur J Biochem. 1995 Jun 1;230(2):797–805. doi: 10.1111/j.1432-1033.1995.0797h.x. [DOI] [PubMed] [Google Scholar]
- Eggens I., Fenderson B., Toyokuni T., Dean B., Stroud M., Hakomori S. Specific interaction between Lex and Lex determinants. A possible basis for cell recognition in preimplantation embryos and in embryonal carcinoma cells. J Biol Chem. 1989 Jun 5;264(16):9476–9484. [PubMed] [Google Scholar]
- Fenderson B. A., Eddy E. M., Hakomori S. Glycoconjugate expression during embryogenesis and its biological significance. Bioessays. 1990 Apr;12(4):173–179. doi: 10.1002/bies.950120406. [DOI] [PubMed] [Google Scholar]
- Fenderson B. A., Zehavi U., Hakomori S. A multivalent lacto-N-fucopentaose III-lysyllysine conjugate decompacts preimplantation mouse embryos, while the free oligosaccharide is ineffective. J Exp Med. 1984 Nov 1;160(5):1591–1596. doi: 10.1084/jem.160.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg B. A., Blennow G., Kristiansson B., Stibler H. Carbohydrate-deficient glycoprotein syndromes: peculiar group of new disorders. Pediatr Neurol. 1993 Jul-Aug;9(4):255–262. doi: 10.1016/0887-8994(93)90060-p. [DOI] [PubMed] [Google Scholar]
- Hammond C., Helenius A. Quality control in the secretory pathway. Curr Opin Cell Biol. 1995 Aug;7(4):523–529. doi: 10.1016/0955-0674(95)80009-3. [DOI] [PubMed] [Google Scholar]
- Ioffe E., Stanley P. Mice lacking N-acetylglucosaminyltransferase I activity die at mid-gestation, revealing an essential role for complex or hybrid N-linked carbohydrates. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):728–732. doi: 10.1073/pnas.91.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeken J., Carchon H., Stibler H. The carbohydrate-deficient glycoprotein syndromes: pre-Golgi and Golgi disorders? Glycobiology. 1993 Oct;3(5):423–428. doi: 10.1093/glycob/3.5.423. [DOI] [PubMed] [Google Scholar]
- Jaeken J., Carchon H. The carbohydrate-deficient glycoprotein syndromes: an overview. J Inherit Metab Dis. 1993;16(5):813–820. doi: 10.1007/BF00714272. [DOI] [PubMed] [Google Scholar]
- Jaeken J., De Cock P., Stibler H., Van Geet C., Kint J., Ramaekers V., Carchon H. Carbohydrate-deficient glycoprotein syndrome type II. J Inherit Metab Dis. 1993;16(6):1041–1041. doi: 10.1007/BF00711522. [DOI] [PubMed] [Google Scholar]
- Jaeken J., Schachter H., Carchon H., De Cock P., Coddeville B., Spik G. Carbohydrate deficient glycoprotein syndrome type II: a deficiency in Golgi localised N-acetyl-glucosaminyltransferase II. Arch Dis Child. 1994 Aug;71(2):123–127. doi: 10.1136/adc.71.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauer R., Lehle L., Hanefeld F., von Figura K. Normal N-oligosaccharyltransferase activity in fibroblasts from patients with carbohydrate-deficient glycoprotein syndrome. J Inherit Metab Dis. 1994;17(5):541–544. doi: 10.1007/BF00711588. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Metzler M., Gertz A., Sarkar M., Schachter H., Schrader J. W., Marth J. D. Complex asparagine-linked oligosaccharides are required for morphogenic events during post-implantation development. EMBO J. 1994 May 1;13(9):2056–2065. doi: 10.1002/j.1460-2075.1994.tb06480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneerselvam K., Freeze H. H. Enzymes involved in the synthesis of mannose-6-phosphate from glucose are normal in carbohydrate deficient glycoprotein syndrome fibroblasts. Biochem Biophys Res Commun. 1995 Mar 17;208(2):517–522. doi: 10.1006/bbrc.1995.1369. [DOI] [PubMed] [Google Scholar]
- Paulson J. C., Colley K. J. Glycosyltransferases. Structure, localization, and control of cell type-specific glycosylation. J Biol Chem. 1989 Oct 25;264(30):17615–17618. [PubMed] [Google Scholar]
- Powell L. D., Paneerselvam K., Vij R., Diaz S., Manzi A., Buist N., Freeze H., Varki A. Carbohydrate-deficient glycoprotein syndrome: not an N-linked oligosaccharide processing defect, but an abnormality in lipid-linked oligosaccharide biosynthesis? J Clin Invest. 1994 Nov;94(5):1901–1909. doi: 10.1172/JCI117540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers V. T., Stibler H., Kint J., Jaeken J. A new variant of the carbohydrate deficient glycoproteins syndrome. J Inherit Metab Dis. 1991;14(3):385–388. doi: 10.1007/BF01811710. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter H. Biosynthetic controls that determine the branching and microheterogeneity of protein-bound oligosaccharides. Biochem Cell Biol. 1986 Mar;64(3):163–181. doi: 10.1139/o86-026. [DOI] [PubMed] [Google Scholar]
- Stibler H., Stephani U., Kutsch U. Carbohydrate-deficient glycoprotein syndrome--a fourth subtype. Neuropediatrics. 1995 Oct;26(5):235–237. doi: 10.1055/s-2007-979762. [DOI] [PubMed] [Google Scholar]
- Stibler H., Westerberg B., Hanefeld F., Hagberg B. Carbohydrate-deficient glycoprotein (CDG) syndrome--a new variant, type III. Neuropediatrics. 1993 Feb;24(1):51–52. doi: 10.1055/s-2008-1071513. [DOI] [PubMed] [Google Scholar]
- Tan J., D'Agostaro A. F., Bendiak B., Reck F., Sarkar M., Squire J. A., Leong P., Schachter H. The human UDP-N-acetylglucosamine: alpha-6-D-mannoside-beta-1,2- N-acetylglucosaminyltransferase II gene (MGAT2). Cloning of genomic DNA, localization to chromosome 14q21, expression in insect cells and purification of the recombinant protein. Eur J Biochem. 1995 Jul 15;231(2):317–328. doi: 10.1111/j.1432-1033.1995.tb20703.x. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Jaeken J. Phosphomannomutase deficiency is a cause of carbohydrate-deficient glycoprotein syndrome type I. FEBS Lett. 1995 Dec 27;377(3):318–320. doi: 10.1016/0014-5793(95)01357-1. [DOI] [PubMed] [Google Scholar]
- Wada Y., Nishikawa A., Okamoto N., Inui K., Tsukamoto H., Okada S., Taniguchi N. Structure of serum transferrin in carbohydrate-deficient glycoprotein syndrome. Biochem Biophys Res Commun. 1992 Dec 15;189(2):832–836. doi: 10.1016/0006-291x(92)92278-6. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Ideo H., Ohkura T., Fukushima K., Yuasa I., Ohno K., Takeshita K. Sugar chains of serum transferrin from patients with carbohydrate deficient glycoprotein syndrome. Evidence of asparagine-N-linked oligosaccharide transfer deficiency. J Biol Chem. 1993 Mar 15;268(8):5783–5789. [PubMed] [Google Scholar]
- Yamashita K., Ohkura T., Ideo H., Ohno K., Kanai M. Electrospray ionization-mass spectrometric analysis of serum transferrin isoforms in patients with carbohydrate-deficient glycoprotein syndrome. J Biochem. 1993 Dec;114(6):766–769. doi: 10.1093/oxfordjournals.jbchem.a124253. [DOI] [PubMed] [Google Scholar]
- Yasugi E., Nakasuji M., Dohi T., Oshima M. Major defect of carbohydrate-deficient-glycoprotein syndrome is not found in the synthesis of dolichyl phosphate or N-acetylglucosaminyl-pyrophosphoryl-dolichol. Biochem Biophys Res Commun. 1994 Apr 29;200(2):816–820. doi: 10.1006/bbrc.1994.1524. [DOI] [PubMed] [Google Scholar]