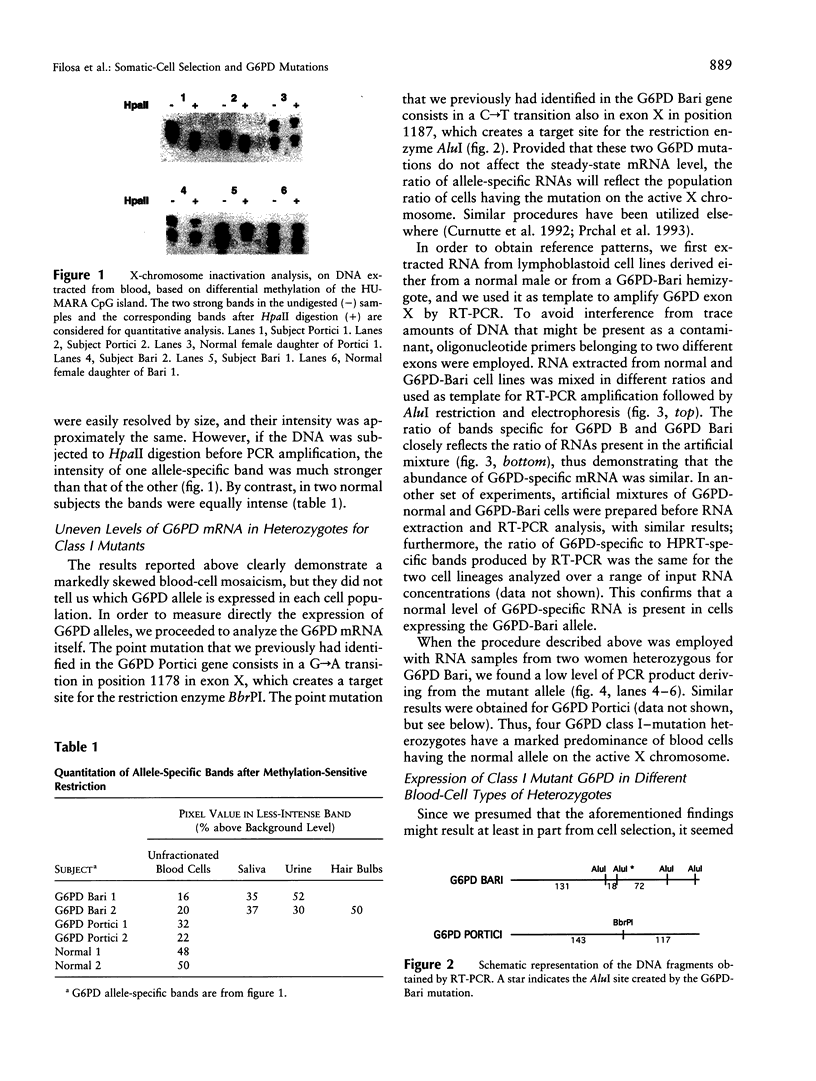
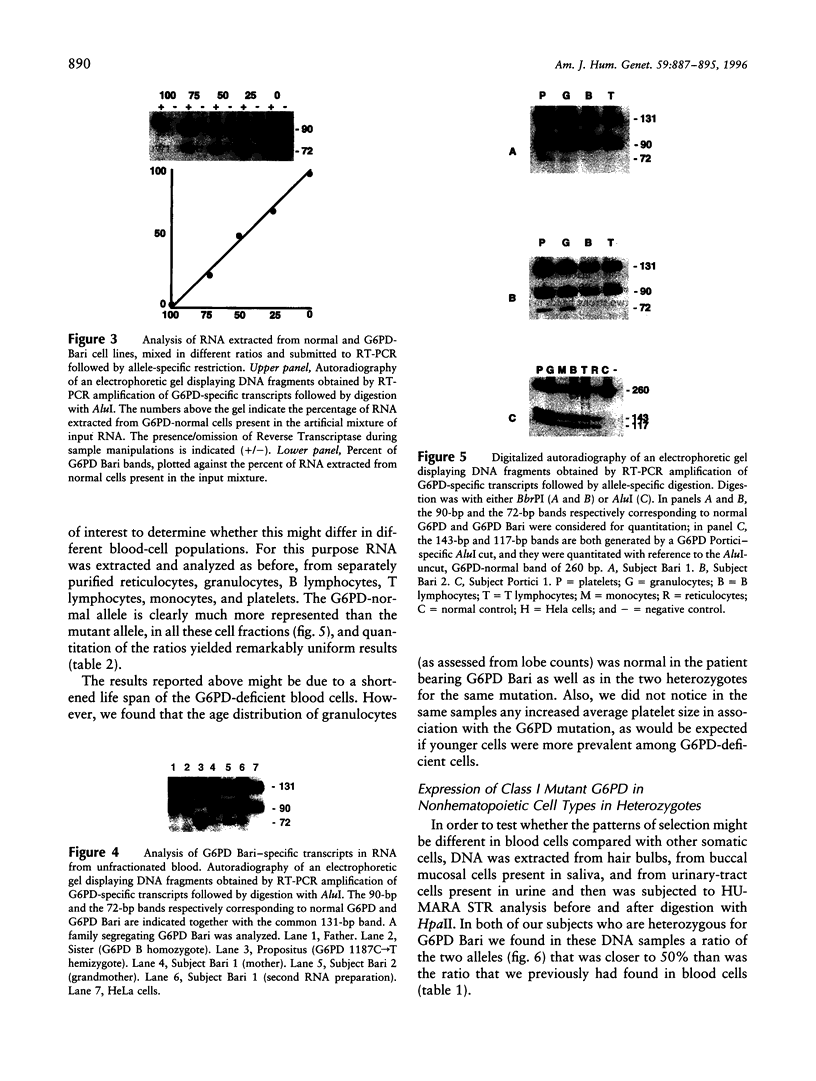
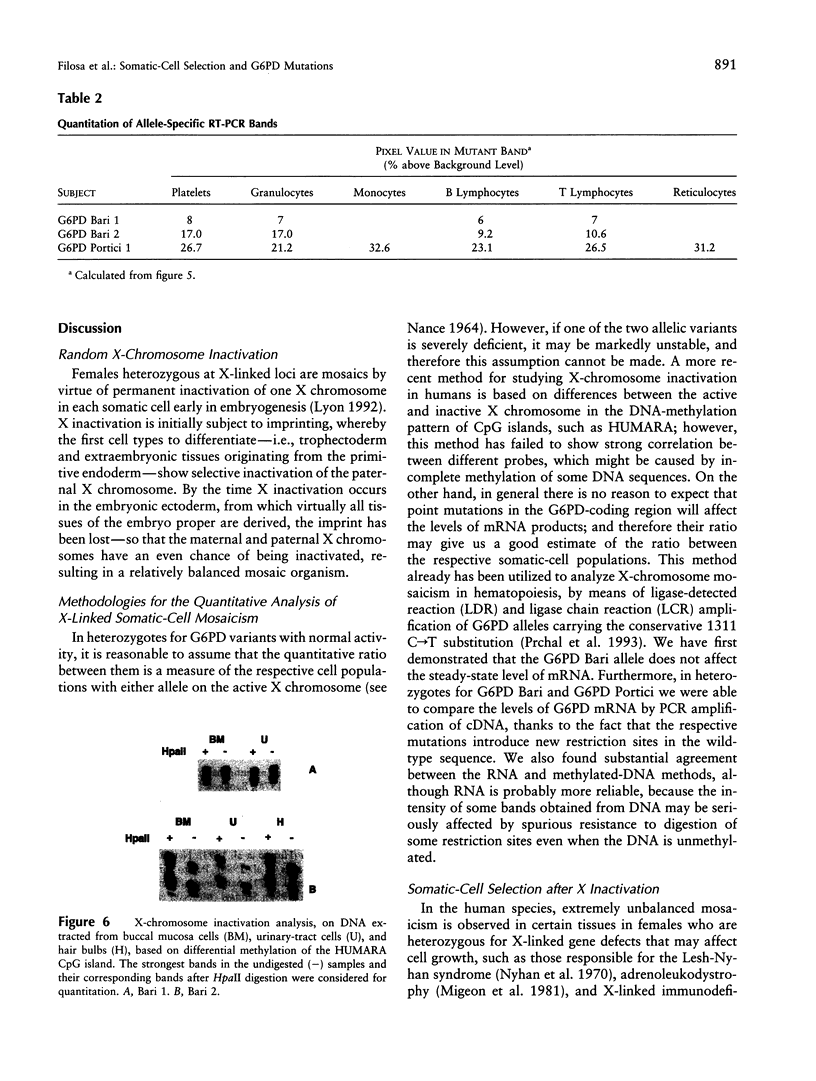
Abstract
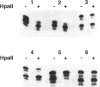
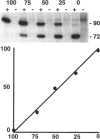
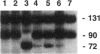
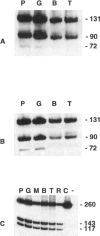
X-chromosome inactivation in mammals is regarded as an essentially random process, but the resulting somatic-cell mosaicism creates the opportunity for cell selection. In most people with red-blood-cell glucose-6-phosphate dehydrogenase (G6PD) deficiency, the enzyme-deficient phenotype is only moderately expressed in nucleated cells. However, in a small subset of hemizygous males who suffer from chronic nonspherocytic hemolytic anemia, the underlying mutations (designated class I) cause more-severe G6PD deficiency, and this might provide an opportunity for selection in heterozygous females during development. In order to test this possibility we have analyzed four heterozygotes for class I G6PD mutations: two with G6PD Portici (1178G-->A) and two with G6PD Bari (1187C-->T). We found that in fractionated blood cell types (including erythroid, myeloid, and lymphoid cell lineages) there was a significant excess of G6PD-normal cells. The significant concordance that we have observed in the degree of imbalance in the different blood-cell lineages indicates that a selective mechanism is likely to operate at the level of pluripotent blood stem cells. Thus, it appears that severe G6PD deficiency affects adversely the proliferation or the survival of nucleated blood cells and that this phenotypic characteristic is critical during hematopoiesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Nachtman R. G., Rosenblatt H. M., Belmont J. W. Application of carrier testing to genetic counseling for X-linked agammaglobulinemia. Am J Hum Genet. 1994 Jan;54(1):25–35. [PMC free article] [PubMed] [Google Scholar]
- Allen R. C., Zoghbi H. Y., Moseley A. B., Rosenblatt H. M., Belmont J. W. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992 Dec;51(6):1229–1239. [PMC free article] [PubMed] [Google Scholar]
- BEUTLER E. GENE INACTIVATION: THE DISTRIBUTION OF GENE PRODUCTS AMONG POPULATIONS OF CELLS IN HETEROZYGOUS HUMANS. Cold Spring Harb Symp Quant Biol. 1964;29:261–271. doi: 10.1101/sqb.1964.029.01.029. [DOI] [PubMed] [Google Scholar]
- Beutler E., Hartman K., Gelbart T., Forman L. G-6-PD Walter Reed: possible insight into "structural" NADP in G-6-PD. Am J Hematol. 1986 Sep;23(1):25–30. doi: 10.1002/ajh.2830230105. [DOI] [PubMed] [Google Scholar]
- Beutler E., Mathai C. K., Smith J. E. Biochemical variants of glucose-6-phosphate dehydrogenase giving rise to congenital nonspherocytic hemolytic disease. Blood. 1968 Feb;31(2):131–150. [PubMed] [Google Scholar]
- Beutler E., Yoshida A. Genetic variation of glucose-6-phosphate dehydrogenase: a catalog and future prospects. Medicine (Baltimore) 1988 Sep;67(5):311–334. doi: 10.1097/00005792-198809000-00003. [DOI] [PubMed] [Google Scholar]
- Brewer G. J., Gall J. C., Honeyman M., Gershowitz H., Shreffler D. C., Dern R. J., Hames C. Inheritance of quantitative expression of erythrocyte glucose-6-phosphate dehydrogenase activity in the negro--a twin study. Biochem Genet. 1967 Jun;1(1):41–53. doi: 10.1007/BF00487735. [DOI] [PubMed] [Google Scholar]
- Cattanach B. M., Williams C. E. Evidence of non-random X chromosome activity in the mouse. Genet Res. 1972 Jun;19(3):229–240. doi: 10.1017/s001667230001449x. [DOI] [PubMed] [Google Scholar]
- Chan T. K., Todd D., Lai M. C. Glucose 6-phosphate dehydrogenase: identity of erythrocyte and leukocyte enzyme with report of a new variant in Chinese. Biochem Genet. 1972 Apr;6(2):119–124. doi: 10.1007/BF00486396. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Colonna-Romano S., Iolascon A., Lippo S., Pinto L., Cutillo S., Battistuzzi G. Genetic heterogeneity at the glucose-6-phosphate dehydrogenase locus in southern Italy: a study on the population of Naples. Hum Genet. 1985;69(3):228–232. doi: 10.1007/BF00293030. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Hopkins P. J., Kuhl W., Beutler E. Studying X inactivation. Lancet. 1992 Mar 21;339(8795):749–749. doi: 10.1016/0140-6736(92)90653-k. [DOI] [PubMed] [Google Scholar]
- Efferth T., Fabry U., Glatte P., Osieka R. Increased induction of apoptosis in mononuclear cells of a glucose-6-phosphate dehydrogenase deficient patient. J Mol Med (Berl) 1995 Jan;73(1):47–49. doi: 10.1007/BF00203619. [DOI] [PubMed] [Google Scholar]
- Elizondo J., Sáenz G. F., Páez C. A., Ramón M., García M., Gutiérrez A., Estrada M. G6PD-Puerto Limón: a new deficient variant of glucose-6-phosphate dehydrogenase associated with congenital nonspherocytic hemolytic anemia. Hum Genet. 1982;62(2):110–112. doi: 10.1007/BF00282295. [DOI] [PubMed] [Google Scholar]
- Engstrom P. F., Beutler E. G-6-PD tripler: a unique variant associated with chronic hemolytic disease. Blood. 1970 Jul;36(1):10–13. [PubMed] [Google Scholar]
- Fairbairn L. J., Cowling G. J., Reipert B. M., Dexter T. M. Suppression of apoptosis allows differentiation and development of a multipotent hemopoietic cell line in the absence of added growth factors. Cell. 1993 Sep 10;74(5):823–832. doi: 10.1016/0092-8674(93)90462-y. [DOI] [PubMed] [Google Scholar]
- Fairbanks V. F., Nepo A. G., Beutler E., Dickson E. R., Honig G. Glucose-6-phosphate dehydrogenase variants: reexamination of G6PD Chicago and Cornell and a new variant (G6PD Pea Ridge) resembling G6PD Chicago. Blood. 1980 Feb;55(2):216–220. [PubMed] [Google Scholar]
- Fialkow P. J. Primordial cell pool size and lineage relationships of five human cell types. Ann Hum Genet. 1973 Jul;37(1):39–48. doi: 10.1111/j.1469-1809.1973.tb01813.x. [DOI] [PubMed] [Google Scholar]
- Filosa S., Cai W., Galanello R., Cao A., de Mattia D., Schettini F., Martini G. A novel single-base mutation in the glucose 6-phosphate dehydrogenase gene is associated with chronic non-spherocytic haemolytic anaemia. Hum Genet. 1994 Nov;94(5):560–562. doi: 10.1007/BF00211027. [DOI] [PubMed] [Google Scholar]
- Filosa S., Calabrò V., Vallone D., Poggi V., Mason P., Pagnini D., Alfinito F., Rotoli B., Martini G., Luzzatto L. Molecular basis of chronic non-spherocytic haemolytic anaemia: a new G6PD variant (393 Arg----His) with abnormal KmG6P and marked in vivo instability. Br J Haematol. 1992 Jan;80(1):111–116. doi: 10.1111/j.1365-2141.1992.tb06409.x. [DOI] [PubMed] [Google Scholar]
- Fraenkel D. G. Selection of Escherichia coli mutants lacking glucose-6-phosphate dehydrogenase or gluconate-6-phosphate dehydrogenase. J Bacteriol. 1968 Apr;95(4):1267–1271. doi: 10.1128/jb.95.4.1267-1271.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H., Miwa S., Takegawa S., Takahashi K., Hirono A., Takizawa T., Morisaki T., Kanno H., Taguchi T., Okamura J. Gd(-) Gifu and Gd(-) Fukuoka. Two new variants of glucose-6-phosphate dehydrogenase found in Japan. Hum Genet. 1984;66(2-3):276–278. doi: 10.1007/BF00286616. [DOI] [PubMed] [Google Scholar]
- Fujii H., Miwa S., Tani K., Takegawa S., Fujinami N., Takahashi K., Nakayama S., Konno M., Sato T. Glucose 6-phosphate dehydrogenase variants: a unique variant (G6PD Kobe) showed an extremely increased affinity for galactose 6-phosphate and a new variant (G6PD Sapporo) resembling G6PD Pea Ridge. Hum Genet. 1981;58(4):405–407. doi: 10.1007/BF00282823. [DOI] [PubMed] [Google Scholar]
- GARTLER S. M., LINDER D. SELECTION IN MAMMALIAN MOSAIC CELL POPULATIONS. Cold Spring Harb Symp Quant Biol. 1964;29:253–260. doi: 10.1101/sqb.1964.029.01.028. [DOI] [PubMed] [Google Scholar]
- Gaetani G. F., Galiano S., Melani C., Miglino M., Forni G. L., Napoli G., Perrone L., Ferraris A. M. A new glucose-6-phosphate dehydrogenase variant with congenital nonspherocytic hemolytic anemia (G6PD Genova). Biochemical characterization and mosaicism expression in the heterozygote. Hum Genet. 1990 Mar;84(4):337–340. doi: 10.1007/BF00196229. [DOI] [PubMed] [Google Scholar]
- Gale R. E., Wheadon H., Linch D. C. X-chromosome inactivation patterns using HPRT and PGK polymorphisms in haematologically normal and post-chemotherapy females. Br J Haematol. 1991 Oct;79(2):193–197. doi: 10.1111/j.1365-2141.1991.tb04521.x. [DOI] [PubMed] [Google Scholar]
- Gvozdev V. A., Gerasimova T. I., Kogan G. L., Braslavskaya OYu Role of the pentose phosphate pathway in metabolism of Drosophila melanogaster elucidated by mutations affecting glucose 6-phosphate and 6-phosphogluconate dehydrogenases. FEBS Lett. 1976 Apr 15;64(1):85–88. doi: 10.1016/0014-5793(76)80255-4. [DOI] [PubMed] [Google Scholar]
- Hall K., Schreeder M. T., Prchal J. T. G6PD Huntsville: a new glucose-6-phosphate dehydrogenase associated with chronic hemolytic anemia. Hum Genet. 1988 May;79(1):90–91. doi: 10.1007/BF00291720. [DOI] [PubMed] [Google Scholar]
- Hirono A., Fujii H., Shima M., Miwa S. G6PD Nara: a new class 1 glucose-6-phosphate dehydrogenase variant with an eight amino acid deletion. Blood. 1993 Dec 1;82(11):3250–3252. [PubMed] [Google Scholar]
- Hughes M. B., Lucchesi J. C. Genetic rescue of a lethal "null" activity allele of 6-phosphogluconate dehydrogenase in Drosophila melanogaster. Science. 1977 Jun 3;196(4294):1114–1115. doi: 10.1126/science.404711. [DOI] [PubMed] [Google Scholar]
- Johnson G. J., Kaplan M. E., Beutler E. G-6-PD Long Prairie: a new glucose-6-phosphate dehydrogenase mutant exhibiting normal sensitivity to inhibition by NADPH and accompanied by nonspherocytic hemolytic anemia. Blood. 1977 Feb;49(2):247–251. [PubMed] [Google Scholar]
- Kahn A., Dao C., Cottreau D., Bilski-Pasquier G. 'Gd(-) Hôtel Dieu': a new G-6PD variant with chronic hemolysis in a Negro patient from Senegal. Hum Genet. 1977 Dec 23;39(3):353–357. doi: 10.1007/BF00295431. [DOI] [PubMed] [Google Scholar]
- Lisker R., Pérez-Briceño R., Beutler E. A new glucose-6-phosphate dehydrogenase variant, Gd(-) Tepic, characterized by moderate enzyme deficiency and mild episodes of hemolytic anemia. Hum Genet. 1985;69(1):19–21. doi: 10.1007/BF00295523. [DOI] [PubMed] [Google Scholar]
- Luzzatto L. Genetics of red cells and susceptibility to malaria. Blood. 1979 Nov;54(5):961–976. [PubMed] [Google Scholar]
- Lyon M. F. Some milestones in the history of X-chromosome inactivation. Annu Rev Genet. 1992;26:16–28. doi: 10.1146/annurev.ge.26.120192.000313. [DOI] [PubMed] [Google Scholar]
- Mentzer W. C., Jr, Warner R., Addiego J., Smith B., Walter T. G6PD San Francisco: a new variant of glucose-6-phosphate dehydrogenase associated with congenital nonspherocytic hemolytic anemia. Blood. 1980 Feb;55(2):195–198. [PubMed] [Google Scholar]
- Migeon B. R., Moser H. W., Moser A. B., Axelman J., Sillence D., Norum R. A. Adrenoleukodystrophy: evidence for X linkage, inactivation, and selection favoring the mutant allele in heterozygous cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5066–5070. doi: 10.1073/pnas.78.8.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. R., Wollman M. R. A new variant of glucose-6-phosphate dehydrogenase deficiency hereditary hemolytic anemia, G6PD Cornell: erythrocyte, leukocyte, and platelet studies. Blood. 1974 Sep;44(3):323–331. [PubMed] [Google Scholar]
- Miwa S., Fujii H., Nakashima K., Miura Y., Yamada K., Hagiwara T., Fukuda M. Three new electrophoretically normal glucose-6-phosphate dehydrogenase variants associated with congenital nonspherocytic hemolytic anemia found in Japan: G6PD Ogikubo, Yokohama, and Akita. Hum Genet. 1978 Nov 24;45(1):11–17. doi: 10.1007/BF00277568. [DOI] [PubMed] [Google Scholar]
- NANCE W. E. GENETIC TESTS WITH A SEX-LINKED MARKER: GLUCOSE-6-PHOSPHATE DEHYDROGENASE. Cold Spring Harb Symp Quant Biol. 1964;29:415–425. doi: 10.1101/sqb.1964.029.01.043. [DOI] [PubMed] [Google Scholar]
- Nogae I., Johnston M. Isolation and characterization of the ZWF1 gene of Saccharomyces cerevisiae, encoding glucose-6-phosphate dehydrogenase. Gene. 1990 Dec 15;96(2):161–169. doi: 10.1016/0378-1119(90)90248-p. [DOI] [PubMed] [Google Scholar]
- Nyhan W. L., Bakay B., Connor J. D., Marks J. F., Keele D. K. Hemizygous expression of glucose-6-phosphate dehydrogenase in erythrocytes of heterozygotes for the Lesch-Nyhan syndrome. Proc Natl Acad Sci U S A. 1970 Jan;65(1):214–218. doi: 10.1073/pnas.65.1.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura H., Morisaki T., Tani K., Kanno H., Tsutsumi H., Takahashi K., Miyamori T., Fujii H., Miwa S. A new glucose-6-phosphate dehydrogenase variant (G6PD Tsukui) associated with congenital hemolytic anemia. Hum Genet. 1988 Apr;78(4):369–371. doi: 10.1007/BF00291738. [DOI] [PubMed] [Google Scholar]
- Pandolfi P. P., Sonati F., Rivi R., Mason P., Grosveld F., Luzzatto L. Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J. 1995 Nov 1;14(21):5209–5215. doi: 10.1002/j.1460-2075.1995.tb00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picat C., Etiemble J., Boivin P., Le Prise P. Y. Gd(-) Rennes, a new deficient variant of glucose-6-phosphate dehydrogenase associated with congenital nonspherocytic hemolytic anemia found in France. Hum Genet. 1980;55(1):125–127. doi: 10.1007/BF00329139. [DOI] [PubMed] [Google Scholar]
- Potocnik A. J., Nielsen P. J., Eichmann K. In vitro generation of lymphoid precursors from embryonic stem cells. EMBO J. 1994 Nov 15;13(22):5274–5283. doi: 10.1002/j.1460-2075.1994.tb06861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prchal J. T., Crist W. M., Malluh A., Vitek A., Tauxe W. N., Carroll A. J. A new glucose-6-phosphate dehydrogenase deficient variant in a patient with Chediak-Higashi syndrome. Blood. 1980 Sep;56(3):476–480. [PubMed] [Google Scholar]
- Prchal J. T., Guan Y. L., Prchal J. F., Barany F. Transcriptional analysis of the active X-chromosome in normal and clonal hematopoiesis. Blood. 1993 Jan 1;81(1):269–271. [PubMed] [Google Scholar]
- Rattazzi M. C., Corash L. M., van Zanen G. E., Jaffé E. R., Piomelli S. G6PD deficiency and chronic hemolysis: four new mutants--relationships between clinical syndrome and enzyme kinetics. Blood. 1971 Aug;38(2):205–218. [PubMed] [Google Scholar]
- Ravindranath Y., Beutler E. Two new variants of glucose-6-phosphate dehydrogenase associated with hereditary non-spherocytic hemolytic anemia: G6PD Wayne and G6PD Huron. Am J Hematol. 1987 Apr;24(4):357–363. doi: 10.1002/ajh.2830240405. [DOI] [PubMed] [Google Scholar]
- Ravindranath Y., Beutler E. Two new variants of glucose-6-phosphate dehydrogenase associated with hereditary non-spherocytic hemolytic anemia: G6PD Wayne and G6PD Huron. Am J Hematol. 1987 Apr;24(4):357–363. doi: 10.1002/ajh.2830240405. [DOI] [PubMed] [Google Scholar]
- Rinaldi A., Filippi G., Siniscalco M. Variability of red cell phenotypes between and within individuals in an unbiased sample of 77 heterozygotes for G6PD deficiency in Sardinia. Am J Hum Genet. 1976 Sep;28(5):496–505. [PMC free article] [PubMed] [Google Scholar]
- Sykes B. C. DNA in heritable disease. Lancet. 1983 Oct 1;2(8353):787–788. doi: 10.1016/s0140-6736(83)92314-0. [DOI] [PubMed] [Google Scholar]
- Sáenz G. F., Chaves M., Berrantes A., Elizondo J., Montero A. G., Yoshida A. A glucose-6-phosphate dehydrogenase variant, Gd(-) Santamaria found in Costa Rica. Acta Haematol. 1984;72(1):37–40. doi: 10.1159/000206354. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Fujii H., Takegawa S., Tani K., Hirono A., Takizawa T., Kawakatsu T., Miwa S. A new glucose-6-phosphate dehydrogenase variant (G6PD Nagano) associated with congenital hemolytic anemia. Hum Genet. 1982;62(4):368–370. doi: 10.1007/BF00304560. [DOI] [PubMed] [Google Scholar]
- Talalak P., Beutler E. G-6PD Bangkok: a new variant found in congenital nonspherocytic hemolytic disease (CNHD). Blood. 1969 May;33(5):772–776. [PubMed] [Google Scholar]
- Thomas D., Cherest H., Surdin-Kerjan Y. Identification of the structural gene for glucose-6-phosphate dehydrogenase in yeast. Inactivation leads to a nutritional requirement for organic sulfur. EMBO J. 1991 Mar;10(3):547–553. doi: 10.1002/j.1460-2075.1991.tb07981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town M., Athanasiou-Metaxa M., Luzzatto L. Intragenic interspecific complementation of glucose 6-phosphate dehydrogenase in human-hamster cell hybrids. Somat Cell Mol Genet. 1990 Mar;16(2):97–108. doi: 10.1007/BF01233040. [DOI] [PubMed] [Google Scholar]
- Vaca G., Ibarra B., Romero F., Olivares N., Cantú J. M., Beutler E. G-6-PD Guadalajara. A new mutant associated with chronic nonspherocytic hemolytic anemia. Hum Genet. 1982;61(2):175–176. doi: 10.1007/BF00274214. [DOI] [PubMed] [Google Scholar]
- Vives Corrons J. L., Feliu E., Pujades M. A., Cardellach F., Rozman C., Carreras A., Jou J. M., Vallespí M. T., Zuazu F. J. Severe-glucose-6-phosphate dehydrogenase (G6PD) deficiency associated with chronic hemolytic anemia, granulocyte dysfunction, and increased susceptibility to infections: description of a new molecular variant (G6PD Barcelona). Blood. 1982 Feb;59(2):428–434. [PubMed] [Google Scholar]
- Vulliamy T. J., D'Urso M., Battistuzzi G., Estrada M., Foulkes N. S., Martini G., Calabro V., Poggi V., Giordano R., Town M. Diverse point mutations in the human glucose-6-phosphate dehydrogenase gene cause enzyme deficiency and mild or severe hemolytic anemia. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5171–5175. doi: 10.1073/pnas.85.14.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. W., Shih L., Hsia D. Y. Characterization of glucose-6-phosphate dehydrogenase among Chinese. Nature. 1965 Dec 25;208(5017):1323–1324. doi: 10.1038/2081323a0. [DOI] [PubMed] [Google Scholar]