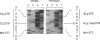Abstract
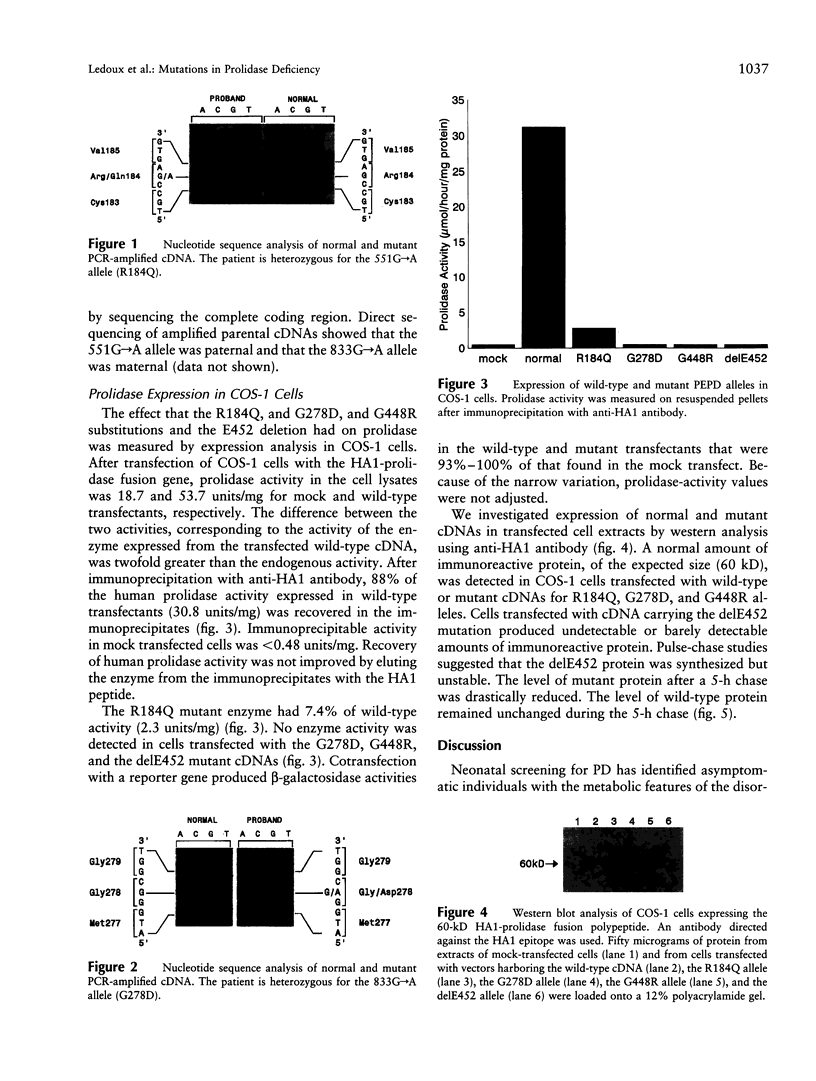
Prolidase (E.C.3.4.13.9) cleaves iminodipeptides. Prolidase deficiency (PD; McKusick 170100) is an autosomal recessive disorder with highly variable penetrance. We have identified two novel alleles in the prolidase gene (PEPD) by direct sequencing of PCR-amplified cDNA from a PD individual asymptomatic at age 11 years: a 551G-->A transition in exon 8 (R184Q) and a 833G-->A transition in exon 12 (G278D). To assess the biochemical phenotypes of these and two previously identified PEPD mutations (G448R and delE452), we have designed a transient-expression system for prolidase in COS-1 cells. The enzyme was expressed as a fusion protein carrying an N-terminal tag, the HA1 epitope of influenza hemagglutinin, allowing its immunological discrimination from the endogenous enzyme with a monoclonal antibody. Expression of the R184Q mutation produced 7.4% of control enzymatic activity whereas the expression of the G278D, G448R, and delE452 mutations produced inactive enzymes. Western analysis of the R184Q, G278D, and G448R prolidases revealed stable immunoreactive material whereas the delE452 prolidase was not detectable. Pulse-chase metabolic labeling of cells followed by immunoprecipitation revealed that the delE452 mutant protein was synthesized but had an increased rate of degradation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazan J. F., Weaver L. H., Roderick S. L., Huber R., Matthews B. W. Sequence and structure comparison suggest that methionine aminopeptidase, prolidase, aminopeptidase P, and creatinase share a common fold. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2473–2477. doi: 10.1073/pnas.91.7.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boright A. P., Scriver C. R., Lancaster G. A., Choy F. Prolidase deficiency: biochemical classification of alleles. Am J Hum Genet. 1989 May;44(5):731–740. [PMC free article] [PubMed] [Google Scholar]
- Butterworth J., Priestman D. A. Presence in human cells and tissues of two prolidases and their alteration in prolidase deficiency. J Inherit Metab Dis. 1985;8(4):193–197. doi: 10.1007/BF01805434. [DOI] [PubMed] [Google Scholar]
- DAVIS N. C., SMITH E. L. Purification and some properties of prolidase of swine kidney. J Biol Chem. 1957 Jan;224(1):261–275. [PubMed] [Google Scholar]
- Endo F., Tanoue A., Nakai H., Hata A., Indo Y., Titani K., Matsuda I. Primary structure and gene localization of human prolidase. J Biol Chem. 1989 Mar 15;264(8):4476–4481. [PubMed] [Google Scholar]
- Isemura M., Hanyu T., Gejyo F., Nakazawa R., Igarashi R., Matsuo S., Ikeda K., Sato Y. Prolidase deficiency with imidodipeptiduria. A familial case with and without clinical symptoms. Clin Chim Acta. 1979 May 2;93(3):401–407. doi: 10.1016/0009-8981(79)90291-2. [DOI] [PubMed] [Google Scholar]
- Jackson S. H., Dennis A. W., Greenberg M. Iminodipeptiduria: a genetic defect in recycling collagen; a method for determining prolidase in erythrocytes. Can Med Assoc J. 1975 Oct 18;113(8):759, 762-3. [PMC free article] [PubMed] [Google Scholar]
- Ledoux P., Scriver C., Hechtman P. Four novel PEPD alleles causing prolidase deficiency. Am J Hum Genet. 1994 Jun;54(6):1014–1021. [PMC free article] [PubMed] [Google Scholar]
- Lemieux B., Auray-Blais C., Giguere R., Shapcott D. Prolidase deficiency: detection of cases by a newborn urinary screening programme. J Inherit Metab Dis. 1984;7 (Suppl 2):145–146. doi: 10.1007/978-94-009-5612-4_47. [DOI] [PubMed] [Google Scholar]
- Mock W. L., Liu Y. Hydrolysis of picolinylprolines by prolidase. A general mechanism for the dual-metal ion containing aminopeptidases. J Biol Chem. 1995 Aug 4;270(31):18437–18446. doi: 10.1074/jbc.270.31.18437. [DOI] [PubMed] [Google Scholar]
- Myara I., Charpentier C., Lemonnier A. Optimal conditions for prolidase assay by proline colorimetric determination: application to iminodipeptiduria. Clin Chim Acta. 1982 Oct 27;125(2):193–205. doi: 10.1016/0009-8981(82)90196-6. [DOI] [PubMed] [Google Scholar]
- Naughten E. R., Proctor S. P., Levy H. L., Coulombe J. T., Ampola M. G. Congenital expression of prolidase defect in prolidase deficiency. Pediatr Res. 1984 Mar;18(3):259–261. doi: 10.1203/00006450-198403000-00008. [DOI] [PubMed] [Google Scholar]
- Powell G. F., Maniscalco R. M. Bound hydroxyproline excretion following gelatin loading in prolidase deficiency. Metabolism. 1976 May;25(5):503–508. doi: 10.1016/0026-0495(76)90003-2. [DOI] [PubMed] [Google Scholar]
- Proia R. L., d'Azzo A., Neufeld E. F. Association of alpha- and beta-subunits during the biosynthesis of beta-hexosaminidase in cultured human fibroblasts. J Biol Chem. 1984 Mar 10;259(5):3350–3354. [PubMed] [Google Scholar]
- Sjöström H., Norén O., Josefsson L. Purification and specificity of pig intestinal prolidase. Biochim Biophys Acta. 1973 Dec 19;327(2):457–470. doi: 10.1016/0005-2744(73)90429-4. [DOI] [PubMed] [Google Scholar]
- Tanoue A., Endo F., Matsuda I. Structural organization of the gene for human prolidase (peptidase D) and demonstration of a partial gene deletion in a patient with prolidase deficiency. J Biol Chem. 1990 Jul 5;265(19):11306–11311. [PubMed] [Google Scholar]
- Umemura S. Studies on a patient with iminodipeptiduria. II. Lack of prolidase activity in blood cells. Physiol Chem Phys. 1978;10(3):279–283. [PubMed] [Google Scholar]