Abstract
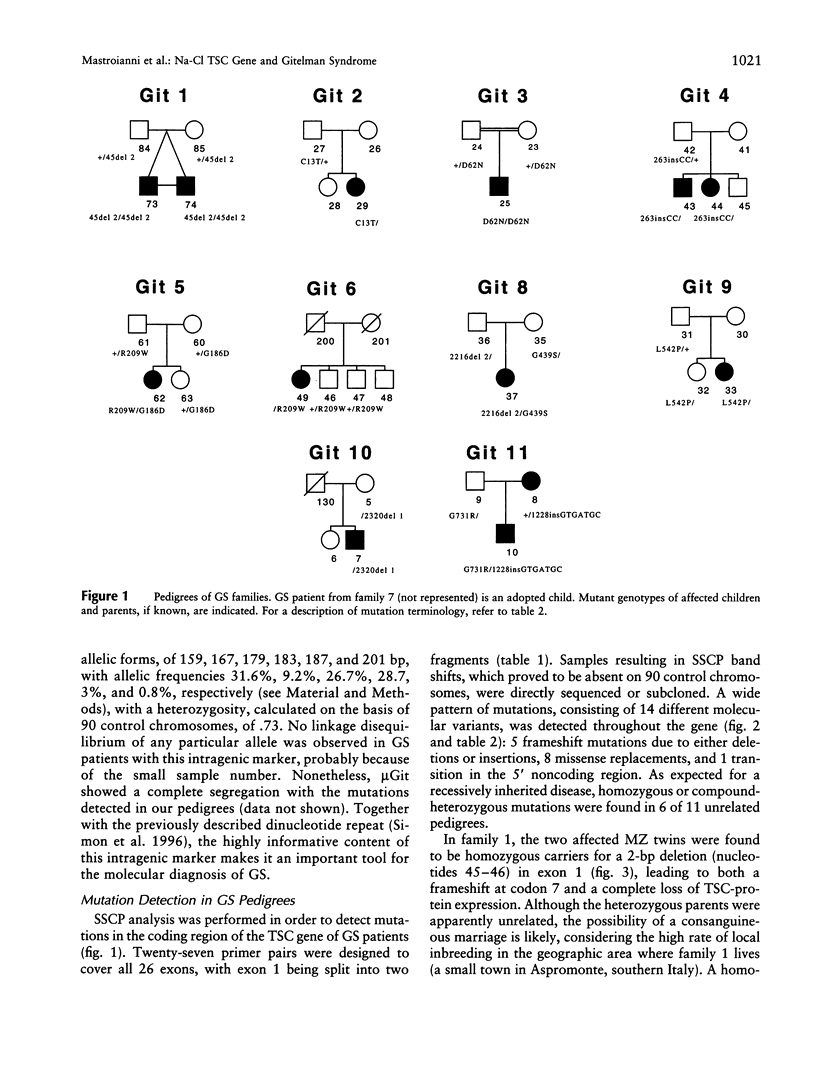
A hereditary defect of the distal tubule accounts for the clinical features of Gitelman syndrome (GS), an autosomal recessive disease characterized by hypokalemia, hypomagnesemia, metabolic alkalosis, and hypocalciuria. Recently, we cloned the cDNA coding for the human Na-Cl thiazide-sensitive cotransporter (TSC; also known as ¿NCCT¿ or ¿SLC12A3¿) as a possible candidate for GS, and Simon et al., independently, described mutations in patients with GS. Now, we show 12 additional mutations consistent with a loss of function of the Na-Cl cotransporter in GS. Two missense replacements, R209W and P349L, are common to both studies and could represent ancient mutations. The other mutations include three deletions, two insertions, and six missense mutations. When all mutations from both studies are considered, missense mutations seem to be more frequently localized within the intracellular domains of the molecule, rather than in transmembrane or extracellular domains. One family, previously reported as a GS form with dominant inheritance, has proved to be recessive, with the affected child being a compound heterozygote. A highly informative intragenic tetranucleotide marker, useful for molecular diagnostic studies, has been identified at the acceptor splice site of exon 9.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bettinelli A., Bianchetti M. G., Borella P., Volpini E., Metta M. G., Basilico E., Selicorni A., Bargellini A., Grassi M. R. Genetic heterogeneity in tubular hypomagnesemia-hypokalemia with hypocalcuria (Gitelman's syndrome). Kidney Int. 1995 Feb;47(2):547–551. doi: 10.1038/ki.1995.68. [DOI] [PubMed] [Google Scholar]
- Bettinelli A., Bianchetti M. G., Girardin E., Caringella A., Cecconi M., Appiani A. C., Pavanello L., Gastaldi R., Isimbaldi C., Lama G. Use of calcium excretion values to distinguish two forms of primary renal tubular hypokalemic alkalosis: Bartter and Gitelman syndromes. J Pediatr. 1992 Jan;120(1):38–43. doi: 10.1016/s0022-3476(05)80594-3. [DOI] [PubMed] [Google Scholar]
- Colussi G., Macaluso M., Brunati C., Minetti L. Calcium metabolism and calciotropic hormone levels in Gitelman's syndrome. Miner Electrolyte Metab. 1994;20(5):294–301. [PubMed] [Google Scholar]
- Gitelman H. J., Graham J. B., Welt L. G. A new familial disorder characterized by hypokalemia and hypomagnesemia. Trans Assoc Am Physicians. 1966;79:221–235. [PubMed] [Google Scholar]
- Kauppinen R., Mustajoki S., Pihlaja H., Peltonen L., Mustajoki P. Acute intermittent porphyria in Finland: 19 mutations in the porphobilinogen deaminase gene. Hum Mol Genet. 1995 Feb;4(2):215–222. doi: 10.1093/hmg/4.2.215. [DOI] [PubMed] [Google Scholar]
- Koch M. C., Steinmeyer K., Lorenz C., Ricker K., Wolf F., Otto M., Zoll B., Lehmann-Horn F., Grzeschik K. H., Jentsch T. J. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science. 1992 Aug 7;257(5071):797–800. doi: 10.1126/science.1379744. [DOI] [PubMed] [Google Scholar]
- Mastroianni N., De Fusco M., Zollo M., Arrigo G., Zuffardi O., Bettinelli A., Ballabio A., Casari G. Molecular cloning, expression pattern, and chromosomal localization of the human Na-Cl thiazide-sensitive cotransporter (SLC12A3). Genomics. 1996 Aug 1;35(3):486–493. doi: 10.1006/geno.1996.0388. [DOI] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Otsu K., Kinsella J. L., Heller P., Froehlich J. P. Sodium dependence of the Na(+)-H+ exchanger in the pre-steady state. Implications for the exchange mechanism. J Biol Chem. 1993 Feb 15;268(5):3184–3193. [PubMed] [Google Scholar]
- Pfaffinger P. J., DeRubeis D. Shaker K+ channel T1 domain self-tetramerizes to a stable structure. J Biol Chem. 1995 Dec 1;270(48):28595–28600. doi: 10.1074/jbc.270.48.28595. [DOI] [PubMed] [Google Scholar]
- Simon D. B., Nelson-Williams C., Bia M. J., Ellison D., Karet F. E., Molina A. M., Vaara I., Iwata F., Cushner H. M., Koolen M. Gitelman's variant of Bartter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet. 1996 Jan;12(1):24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- Sutton R. A., Mavichak V., Halabe A., Wilkins G. E. Bartter's syndrome: evidence suggesting a distal tubular defect in a hypocalciuric variant of the syndrome. Miner Electrolyte Metab. 1992;18(1):43–51. [PubMed] [Google Scholar]