Abstract
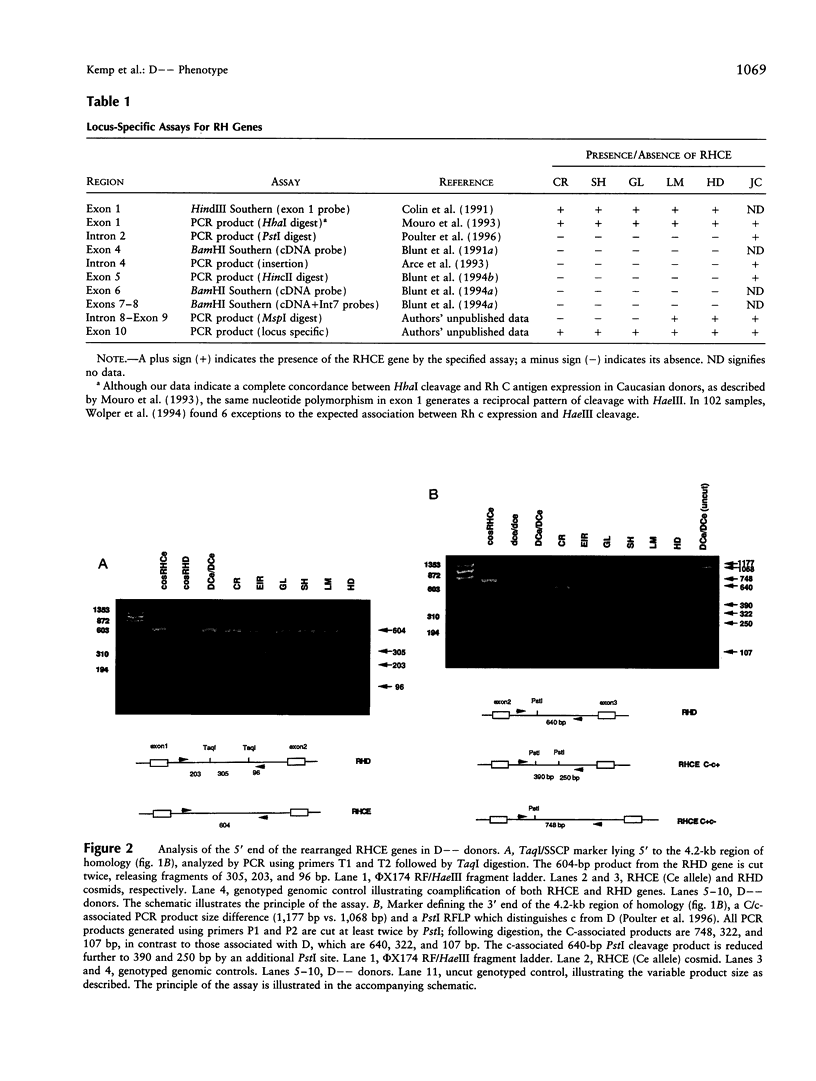
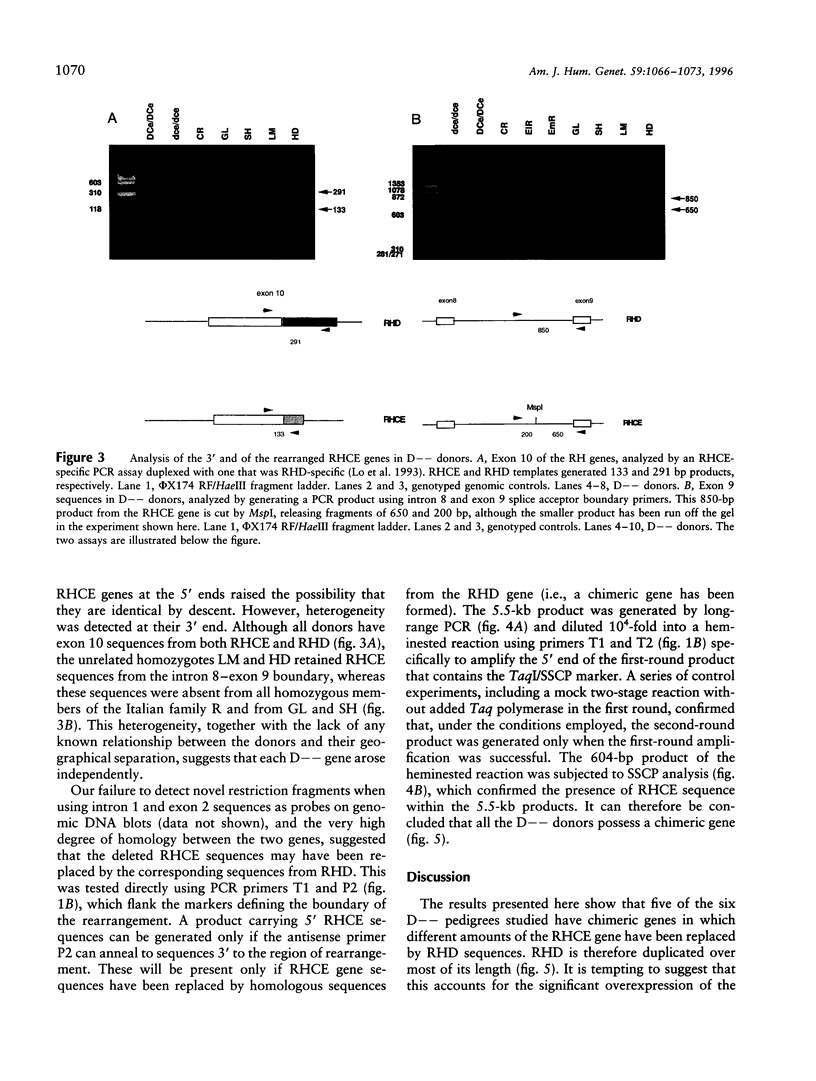
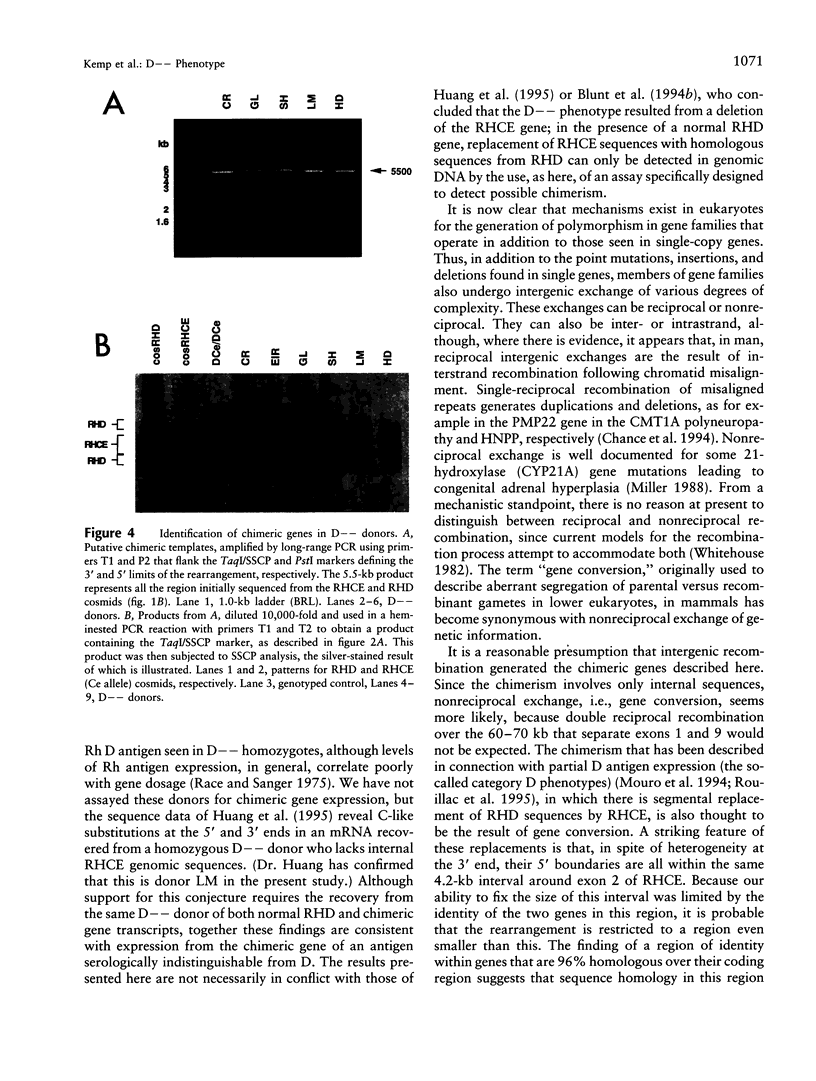
We have studied the arrangement of Rh (rhesus) genes in donors who are completely null for the products of one of them, RHCE. We show that five of six homozygous individuals with the so-called Rh D-- phenotype, who express no red-cell antigens of the C/c and E/e series, have rearranged RHCE genes in which internal sequences have been replaced by the corresponding sequences from RHD. Moreover, although there is heterogeneity at the 3' end, the 5' boundary of this chimerism is within the same small interval around exon 2. This interval is characterized by an exceptionally high degree of sequence homology between RHCE and RHD, a high density of dispersed repetitive elements, and the presence of an alternating purine-pyrimidine copolymer tract. We suggest that these features may explain the mechanistic basis for the origin of the rearrangement.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arce M. A., Thompson E. S., Wagner S., Coyne K. E., Ferdman B. A., Lublin D. M. Molecular cloning of RhD cDNA derived from a gene present in RhD-positive, but not RhD-negative individuals. Blood. 1993 Jul 15;82(2):651–655. [PubMed] [Google Scholar]
- Armour J. A., Wong Z., Wilson V., Royle N. J., Jeffreys A. J. Sequences flanking the repeat arrays of human minisatellites: association with tandem and dispersed repeat elements. Nucleic Acids Res. 1989 Jul 11;17(13):4925–4935. doi: 10.1093/nar/17.13.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCHANAN D. I. Blood genotypes-D-/-D- and Cde/-D-; transfusion therapy and some effects of multiple pregnancy. Am J Clin Pathol. 1956 Jan;26(1):21–28. doi: 10.1093/ajcp/26.1.21. [DOI] [PubMed] [Google Scholar]
- Blunt T., Daniels G., Carritt B. Serotype switching in a partially deleted RHD gene. Vox Sang. 1994;67(4):397–401. doi: 10.1111/j.1423-0410.1994.tb01280.x. [DOI] [PubMed] [Google Scholar]
- Blunt T., Steers F., Daniels G., Carritt B. Lack of RH C/E expression in the Rhesus D--phenotype is the result of a gene deletion. Ann Hum Genet. 1994 Jan;58(Pt 1):19–24. doi: 10.1111/j.1469-1809.1994.tb00722.x. [DOI] [PubMed] [Google Scholar]
- Boehm T., Mengle-Gaw L., Kees U. R., Spurr N., Lavenir I., Forster A., Rabbitts T. H. Alternating purine-pyrimidine tracts may promote chromosomal translocations seen in a variety of human lymphoid tumours. EMBO J. 1989 Sep;8(9):2621–2631. doi: 10.1002/j.1460-2075.1989.tb08402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carritt B., Blunt T., Avent N., Daniels G., Steers F. Rh null phenotypes are not due to a gross deletion and can occur on different Rh genetic backgrounds. Ann Hum Genet. 1993 Oct;57(Pt 4):273–279. doi: 10.1111/j.1469-1809.1993.tb00900.x. [DOI] [PubMed] [Google Scholar]
- Chance P. F., Abbas N., Lensch M. W., Pentao L., Roa B. B., Patel P. I., Lupski J. R. Two autosomal dominant neuropathies result from reciprocal DNA duplication/deletion of a region on chromosome 17. Hum Mol Genet. 1994 Feb;3(2):223–228. doi: 10.1093/hmg/3.2.223. [DOI] [PubMed] [Google Scholar]
- Chérif-Zahar B., Le Van Kim C., Rouillac C., Raynal V., Cartron J. P., Colin Y. Organization of the gene (RHCE) encoding the human blood group RhCcEe antigens and characterization of the promoter region. Genomics. 1994 Jan 1;19(1):68–74. doi: 10.1006/geno.1994.1014. [DOI] [PubMed] [Google Scholar]
- Chérif-Zahar B., Raynal V., D'Ambrosio A. M., Cartron J. P., Colin Y. Molecular analysis of the structure and expression of the RH locus in individuals with D--, Dc-, and DCw- gene complexes. Blood. 1994 Dec 15;84(12):4354–4360. [PubMed] [Google Scholar]
- Chérif-Zahar B., Raynal V., Le Van Kim C., D'Ambrosio A. M., Bailly P., Cartron J. P., Colin Y. Structure and expression of the RH locus in the Rh-deficiency syndrome. Blood. 1993 Jul 15;82(2):656–662. [PubMed] [Google Scholar]
- Colin Y., Chérif-Zahar B., Le Van Kim C., Raynal V., Van Huffel V., Cartron J. P. Genetic basis of the RhD-positive and RhD-negative blood group polymorphism as determined by Southern analysis. Blood. 1991 Nov 15;78(10):2747–2752. [PubMed] [Google Scholar]
- Contreras M., Armitage S., Daniels G. L., Tippett P. Homozygous.D. Vox Sang. 1979;36(2):81–84. doi: 10.1111/j.1423-0410.1979.tb04403.x. [DOI] [PubMed] [Google Scholar]
- Huang C. H., Reid M. E., Chen Y. Identification of a partial internal deletion in the RH locus causing the human erythrocyte D--phenotype. Blood. 1995 Jul 15;86(2):784–790. [PubMed] [Google Scholar]
- Le van Kim C., Mouro I., Chérif-Zahar B., Raynal V., Cherrier C., Cartron J. P., Colin Y. Molecular cloning and primary structure of the human blood group RhD polypeptide. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10925–10929. doi: 10.1073/pnas.89.22.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman M. A., Goldstein J. L., Russell D. W., Brown M. S. Duplication of seven exons in LDL receptor gene caused by Alu-Alu recombination in a subject with familial hypercholesterolemia. Cell. 1987 Mar 13;48(5):827–835. doi: 10.1016/0092-8674(87)90079-1. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Russell D. W., Goldstein J. L., Brown M. S. Alu-Alu recombination deletes splice acceptor sites and produces secreted low density lipoprotein receptor in a subject with familial hypercholesterolemia. J Biol Chem. 1987 Mar 5;262(7):3354–3361. [PubMed] [Google Scholar]
- Lo Y. M., Bowell P. J., Selinger M., Mackenzie I. Z., Chamberlain P., Gillmer M. D., Littlewood T. J., Fleming K. A., Wainscoat J. S. Prenatal determination of fetal RhD status by analysis of peripheral blood of rhesus negative mothers. Lancet. 1993 May 1;341(8853):1147–1148. doi: 10.1016/0140-6736(93)93161-s. [DOI] [PubMed] [Google Scholar]
- Markert M. L., Hutton J. J., Wiginton D. A., States J. C., Kaufman R. E. Adenosine deaminase (ADA) deficiency due to deletion of the ADA gene promoter and first exon by homologous recombination between two Alu elements. J Clin Invest. 1988 May;81(5):1323–1327. doi: 10.1172/JCI113458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. L. Gene conversions, deletions, and polymorphisms in congenital adrenal hyperplasia. Am J Hum Genet. 1988 Jan;42(1):4–7. [PMC free article] [PubMed] [Google Scholar]
- Mora M. L., Botti G., Lomas C. Ten homozygous-D-individuals in one Italian village. Hum Hered. 1990;40(5):278–284. doi: 10.1159/000153945. [DOI] [PubMed] [Google Scholar]
- Mouro I., Colin Y., Chérif-Zahar B., Cartron J. P., Le Van Kim C. Molecular genetic basis of the human Rhesus blood group system. Nat Genet. 1993 Sep;5(1):62–65. doi: 10.1038/ng0993-62. [DOI] [PubMed] [Google Scholar]
- Mouro I., Le Van Kim C., Rouillac C., van Rhenen D. J., Le Pennec P. Y., Bailly P., Cartron J. P., Colin Y. Rearrangements of the blood group RhD gene associated with the DVI category phenotype. Blood. 1994 Feb 15;83(4):1129–1135. [PubMed] [Google Scholar]
- Olafsdóttir S., Jensson O., Thordarson G., Sigurdardóttir S. An unusual Rhesus haplotype, --D--, in Iceland. Forensic Sci Int. 1983 Aug-Sep;22(2-3):183–187. doi: 10.1016/0379-0738(83)90012-9. [DOI] [PubMed] [Google Scholar]
- Poulter M., Kemp T. J., Carritt B. DNA-based rhesus typing: simultaneous determination of RHC and RHD status using the polymerase chain reaction. Vox Sang. 1996;70(3):164–168. doi: 10.1111/j.1423-0410.1996.tb01316.x. [DOI] [PubMed] [Google Scholar]
- Rouillac C., Colin Y., Hughes-Jones N. C., Beolet M., D'Ambrosio A. M., Cartron J. P., Le Van Kim C. Transcript analysis of D category phenotypes predicts hybrid Rh D-CE-D proteins associated with alteration of D epitopes. Blood. 1995 May 15;85(10):2937–2944. [PubMed] [Google Scholar]
- Rouyer F., Simmler M. C., Page D. C., Weissenbach J. A sex chromosome rearrangement in a human XX male caused by Alu-Alu recombination. Cell. 1987 Nov 6;51(3):417–425. doi: 10.1016/0092-8674(87)90637-4. [DOI] [PubMed] [Google Scholar]
- Simsek S., de Jong C. A., Cuijpers H. T., Bleeker P. M., Westers T. M., Overbeeke M. A., Goldschmeding R., van der Schoot C. E., von dem Borne A. E. Sequence analysis of cDNA derived from reticulocyte mRNAs coding for Rh polypeptides and demonstration of E/e and C/c polymorphisms. Vox Sang. 1994;67(2):203–209. doi: 10.1111/j.1423-0410.1994.tb01661.x. [DOI] [PubMed] [Google Scholar]
- Stringer J. R. Recombination between poly[d(GT).d(CA)] sequences in simian virus 40-infected cultured cells. Mol Cell Biol. 1985 Jun;5(6):1247–1259. doi: 10.1128/mcb.5.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treco D., Arnheim N. The evolutionarily conserved repetitive sequence d(TG.AC)n promotes reciprocal exchange and generates unusual recombinant tetrads during yeast meiosis. Mol Cell Biol. 1986 Nov;6(11):3934–3947. doi: 10.1128/mcb.6.11.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter L. C., Hyland C. A., Saul A. Refining the DNA polymorphisms that associate with the rhesus c phenotype. Blood. 1994 Aug 1;84(3):985–986. [PubMed] [Google Scholar]