Abstract
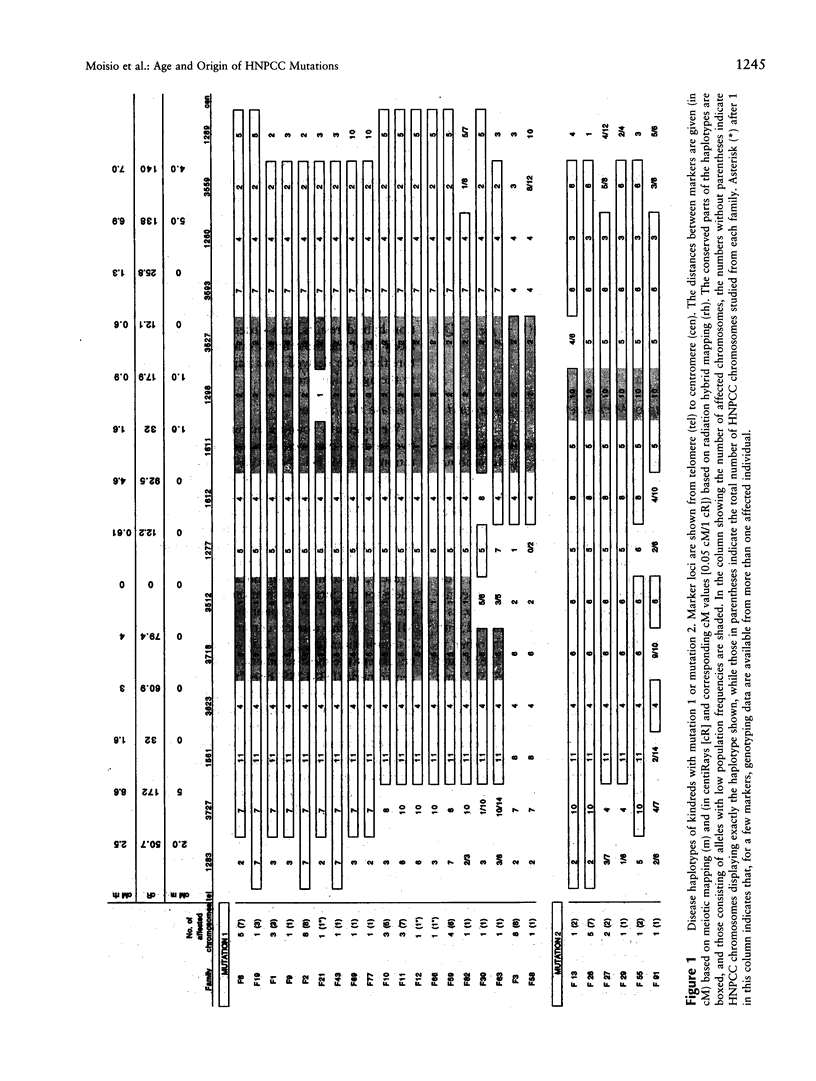
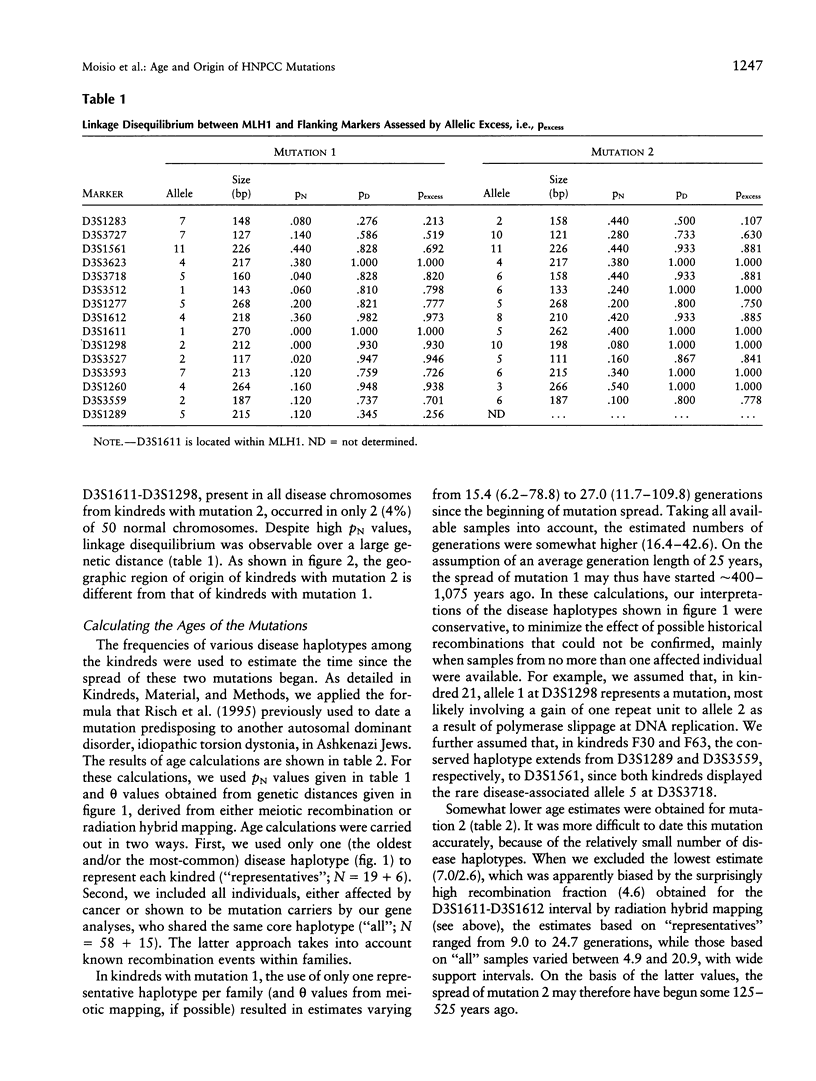
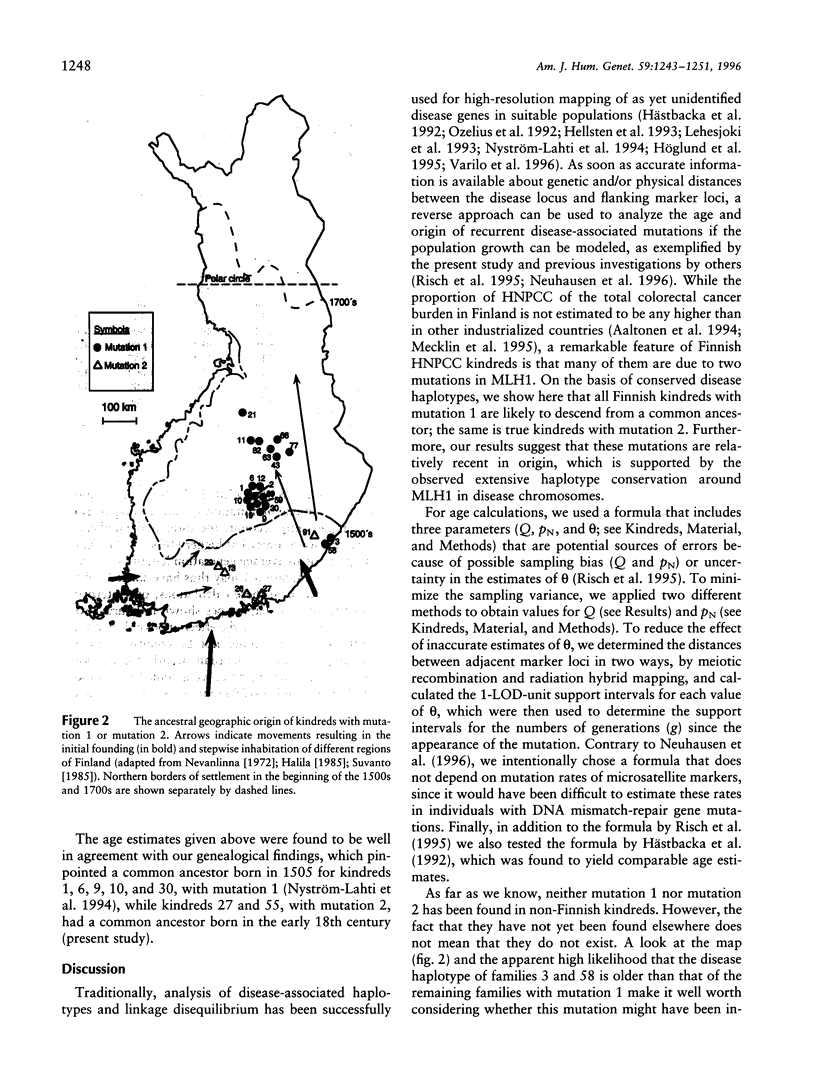
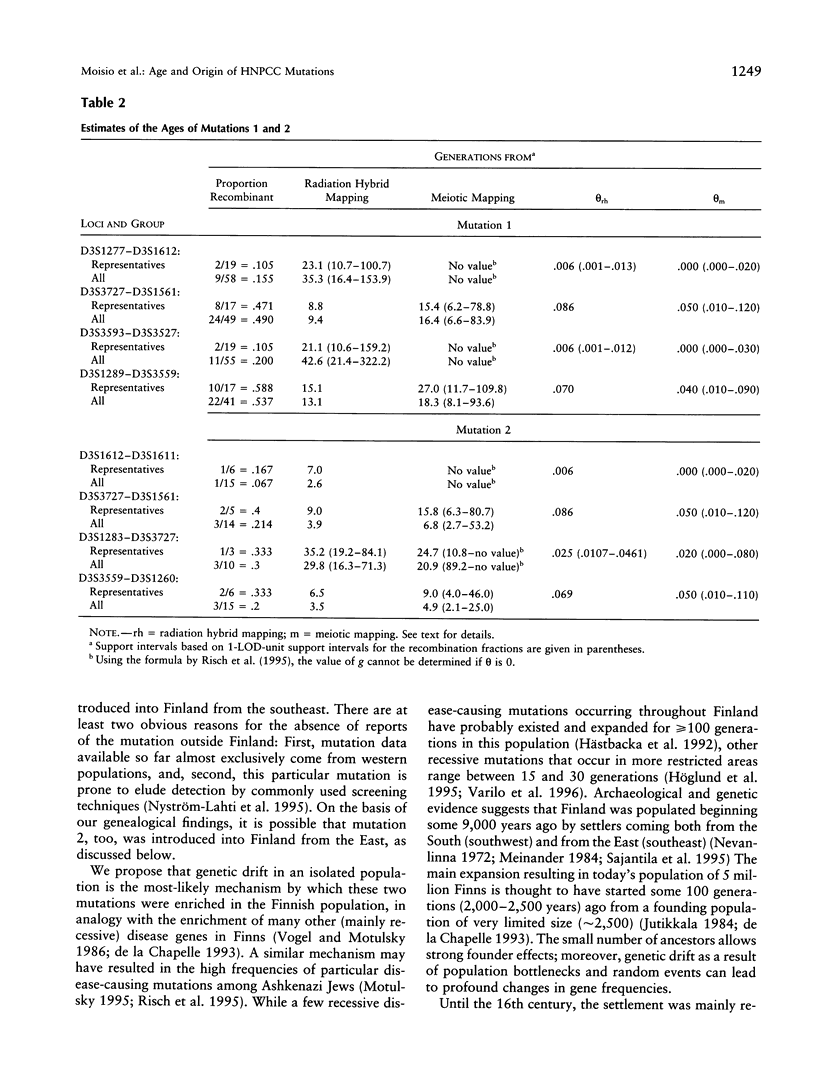
Two mutations in the DNA mismatch repair gene MLH1, referred to as mutations 1 and 2, are frequent among Finnish kindreds with hereditary nonpolyposis colorectal cancer (HNPCC). In order to assess the ages and origins of these mutations, we constructed a map of 15 microsatellite markers around MLH1 and used this information in haplotype analyses of 19 kindreds with mutation 1 and 6 kindreds with mutation 2. All kindreds with mutation 1 showed a single allele for the intragenic marker D3S1611 that was not observed on any unaffected chromosome. They also shared portions of a haplotype of 4-15 markers encompassing 2.0-19.0 cM around MLH1. All kindreds with mutation 2 shared another allele for D3S1611 and a conserved haplotype of 5-14 markers spanning 2.0-15.0 cM around MLH1. The degree of haplotype conservation was used to estimate the ages of these two mutations. While some recessive disease genes have been estimated to have existed and spread for as long as thousands of generations worldwide and hundreds of generations in the Finnish population, our analyses suggest that the spread of mutation 1 started 16-43 generations (400-1,075 years) ago and that of mutation 2 some 5-21 generations (125-525 years) ago. These datings are compatible with our genealogical results identifying a common ancestor born in the 16th and 18th century, respectively. Overall, our results indicate that all Finnish kindreds studied to date showing either mutation 1 or mutation 2 are due to single ancestral founding mutations relatively recent in origin in the population. Alternatively, the mutations arose elsewhere earlier and were introduced in Finland more recently.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Sankila R., Mecklin J. P., Järvinen H., Pukkala E., Peltomäki P., de la Chapelle A. A novel approach to estimate the proportion of hereditary nonpolyposis colorectal cancer of total colorectal cancer burden. Cancer Detect Prev. 1994;18(1):57–63. [PubMed] [Google Scholar]
- Aarnio M., Mecklin J. P., Aaltonen L. A., Nyström-Lahti M., Järvinen H. J. Life-time risk of different cancers in hereditary non-polyposis colorectal cancer (HNPCC) syndrome. Int J Cancer. 1995 Dec 20;64(6):430–433. doi: 10.1002/ijc.2910640613. [DOI] [PubMed] [Google Scholar]
- Boehnke M., Lange K., Cox D. R. Statistical methods for multipoint radiation hybrid mapping. Am J Hum Genet. 1991 Dec;49(6):1174–1188. [PMC free article] [PubMed] [Google Scholar]
- Bronner C. E., Baker S. M., Morrison P. T., Warren G., Smith L. G., Lescoe M. K., Kane M., Earabino C., Lipford J., Lindblom A. Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature. 1994 Mar 17;368(6468):258–261. doi: 10.1038/368258a0. [DOI] [PubMed] [Google Scholar]
- Cox D. R., Burmeister M., Price E. R., Kim S., Myers R. M. Radiation hybrid mapping: a somatic cell genetic method for constructing high-resolution maps of mammalian chromosomes. Science. 1990 Oct 12;250(4978):245–250. doi: 10.1126/science.2218528. [DOI] [PubMed] [Google Scholar]
- Dib C., Fauré S., Fizames C., Samson D., Drouot N., Vignal A., Millasseau P., Marc S., Hazan J., Seboun E. A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature. 1996 Mar 14;380(6570):152–154. doi: 10.1038/380152a0. [DOI] [PubMed] [Google Scholar]
- Froggatt N. J., Joyce J. A., Davies R., Gareth D., Evans R., Ponder B. A., Barton D. E., Maher E. R. A frequent hMSH2 mutation in hereditary non-polyposis colon cancer syndrome. Lancet. 1995 Mar 18;345(8951):727–727. doi: 10.1016/s0140-6736(95)90900-1. [DOI] [PubMed] [Google Scholar]
- Hellsten E., Vesa J., Speer M. C., Mäkelä T. P., Järvelä I., Alitalo K., Ott J., Peltonen L. Refined assignment of the infantile neuronal ceroid lipofuscinosis (INCL, CLN1) locus at 1p32: incorporation of linkage disequilibrium in multipoint analysis. Genomics. 1993 Jun;16(3):720–725. doi: 10.1006/geno.1993.1253. [DOI] [PubMed] [Google Scholar]
- Hästbacka J., de la Chapelle A., Kaitila I., Sistonen P., Weaver A., Lander E. Linkage disequilibrium mapping in isolated founder populations: diastrophic dysplasia in Finland. Nat Genet. 1992 Nov;2(3):204–211. doi: 10.1038/ng1192-204. [DOI] [PubMed] [Google Scholar]
- Höglund P., Sistonen P., Norio R., Holmberg C., Dimberg A., Gustavson K. H., de la Chapelle A., Kere J. Fine mapping of the congenital chloride diarrhea gene by linkage disequilibrium. Am J Hum Genet. 1995 Jul;57(1):95–102. [PMC free article] [PubMed] [Google Scholar]
- Kerem B., Rommens J. M., Buchanan J. A., Markiewicz D., Cox T. K., Chakravarti A., Buchwald M., Tsui L. C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989 Sep 8;245(4922):1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Leach F. S., Nicolaides N. C., Papadopoulos N., Liu B., Jen J., Parsons R., Peltomäki P., Sistonen P., Aaltonen L. A., Nyström-Lahti M. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell. 1993 Dec 17;75(6):1215–1225. doi: 10.1016/0092-8674(93)90330-s. [DOI] [PubMed] [Google Scholar]
- Lehesjoki A. E., Koskiniemi M., Norio R., Tirrito S., Sistonen P., Lander E., de la Chapelle A. Localization of the EPM1 gene for progressive myoclonus epilepsy on chromosome 21: linkage disequilibrium allows high resolution mapping. Hum Mol Genet. 1993 Aug;2(8):1229–1234. doi: 10.1093/hmg/2.8.1229. [DOI] [PubMed] [Google Scholar]
- Mecklin J. P., Järvinen H. J., Hakkiluoto A., Hallikas H., Hiltunen K. M., Härkönen N., Kellokumpu I., Laitinen S., Ovaska J., Tulikoura J. Frequency of hereditary nonpolyposis colorectal cancer. A prospective multicenter study in Finland. Dis Colon Rectum. 1995 Jun;38(6):588–593. doi: 10.1007/BF02054116. [DOI] [PubMed] [Google Scholar]
- Morral N., Bertranpetit J., Estivill X., Nunes V., Casals T., Giménez J., Reis A., Varon-Mateeva R., Macek M., Jr, Kalaydjieva L. The origin of the major cystic fibrosis mutation (delta F508) in European populations. Nat Genet. 1994 Jun;7(2):169–175. doi: 10.1038/ng0694-169. [DOI] [PubMed] [Google Scholar]
- Motulsky A. G. Jewish diseases and origins. Nat Genet. 1995 Feb;9(2):99–101. doi: 10.1038/ng0295-99. [DOI] [PubMed] [Google Scholar]
- Neuhausen S. L., Mazoyer S., Friedman L., Stratton M., Offit K., Caligo A., Tomlinson G., Cannon-Albright L., Bishop T., Kelsell D. Haplotype and phenotype analysis of six recurrent BRCA1 mutations in 61 families: results of an international study. Am J Hum Genet. 1996 Feb;58(2):271–280. [PMC free article] [PubMed] [Google Scholar]
- Nevanlinna H. R. The Finnish population structure. A genetic and genealogical study. Hereditas. 1972;71(2):195–236. doi: 10.1111/j.1601-5223.1972.tb01021.x. [DOI] [PubMed] [Google Scholar]
- Nicolaides N. C., Papadopoulos N., Liu B., Wei Y. F., Carter K. C., Ruben S. M., Rosen C. A., Haseltine W. A., Fleischmann R. D., Fraser C. M. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature. 1994 Sep 1;371(6492):75–80. doi: 10.1038/371075a0. [DOI] [PubMed] [Google Scholar]
- Nyström-Lahti M., Kristo P., Nicolaides N. C., Chang S. Y., Aaltonen L. A., Moisio A. L., Järvinen H. J., Mecklin J. P., Kinzler K. W., Vogelstein B. Founding mutations and Alu-mediated recombination in hereditary colon cancer. Nat Med. 1995 Nov;1(11):1203–1206. doi: 10.1038/nm1195-1203. [DOI] [PubMed] [Google Scholar]
- Nyström-Lahti M., Sistonen P., Mecklin J. P., Pylkkänen L., Aaltonen L. A., Järvinen H., Weissenbach J., de la Chapelle A., Peltomäki P. Close linkage to chromosome 3p and conservation of ancestral founding haplotype in hereditary nonpolyposis colorectal cancer families. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6054–6058. doi: 10.1073/pnas.91.13.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström-Lahti M., Wu Y., Moisio A. L., Hofstra R. M., Osinga J., Mecklin J. P., Järvinen H. J., Leisti J., Buys C. H., de la Chapelle A. DNA mismatch repair gene mutations in 55 kindreds with verified or putative hereditary non-polyposis colorectal cancer. Hum Mol Genet. 1996 Jun;5(6):763–769. doi: 10.1093/hmg/5.6.763. [DOI] [PubMed] [Google Scholar]
- Ozelius L. J., Kramer P. L., de Leon D., Risch N., Bressman S. B., Schuback D. E., Brin M. F., Kwiatkowski D. J., Burke R. E., Gusella J. F. Strong allelic association between the torsion dystonia gene (DYT1) andloci on chromosome 9q34 in Ashkenazi Jews. Am J Hum Genet. 1992 Mar;50(3):619–628. [PMC free article] [PubMed] [Google Scholar]
- Peltomäki P., Aaltonen L. A., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Green J. S., Jass J. R., Weber J. L., Leach F. S. Genetic mapping of a locus predisposing to human colorectal cancer. Science. 1993 May 7;260(5109):810–812. doi: 10.1126/science.8484120. [DOI] [PubMed] [Google Scholar]
- Pirastu M., Galanello R., Doherty M. A., Tuveri T., Cao A., Kan Y. W. The same beta-globin gene mutation is present on nine different beta-thalassemia chromosomes in a Sardinian population. Proc Natl Acad Sci U S A. 1987 May;84(9):2882–2885. doi: 10.1073/pnas.84.9.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N., de Leon D., Ozelius L., Kramer P., Almasy L., Singer B., Fahn S., Breakefield X., Bressman S. Genetic analysis of idiopathic torsion dystonia in Ashkenazi Jews and their recent descent from a small founder population. Nat Genet. 1995 Feb;9(2):152–159. doi: 10.1038/ng0295-152. [DOI] [PubMed] [Google Scholar]
- Sajantila A., Lahermo P., Anttinen T., Lukka M., Sistonen P., Savontaus M. L., Aula P., Beckman L., Tranebjaerg L., Gedde-Dahl T. Genes and languages in Europe: an analysis of mitochondrial lineages. Genome Res. 1995 Aug;5(1):42–52. doi: 10.1101/gr.5.1.42. [DOI] [PubMed] [Google Scholar]
- Varilo T., Savukoski M., Norio R., Santavuori P., Peltonen L., Järvelä I. The age of human mutation: genealogical and linkage disequilibrium analysis of the CLN5 mutation in the Finnish population. Am J Hum Genet. 1996 Mar;58(3):506–512. [PMC free article] [PubMed] [Google Scholar]
- Vasen H. F., Mecklin J. P., Khan P. M., Lynch H. T. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum. 1991 May;34(5):424–425. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- de la Chapelle A. Disease gene mapping in isolated human populations: the example of Finland. J Med Genet. 1993 Oct;30(10):857–865. doi: 10.1136/jmg.30.10.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Chapelle A., Peltomäki P. Genetics of hereditary colon cancer. Annu Rev Genet. 1995;29:329–348. doi: 10.1146/annurev.ge.29.120195.001553. [DOI] [PubMed] [Google Scholar]



