Abstract
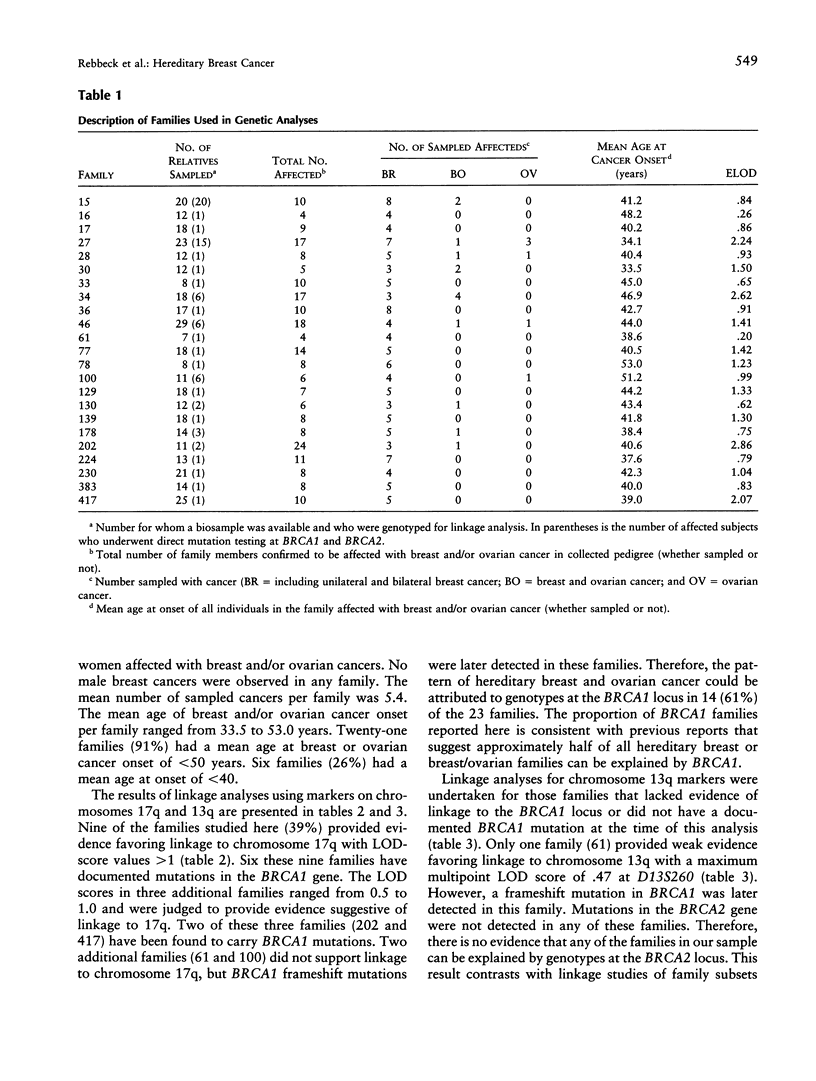
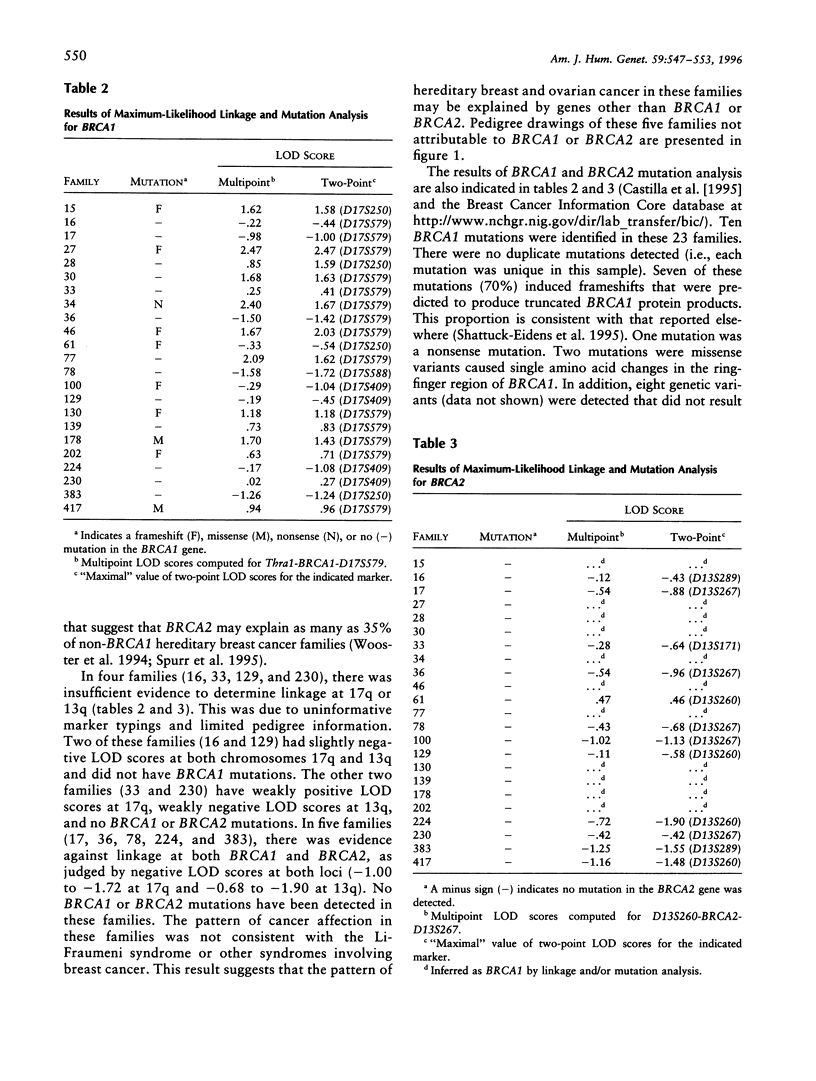
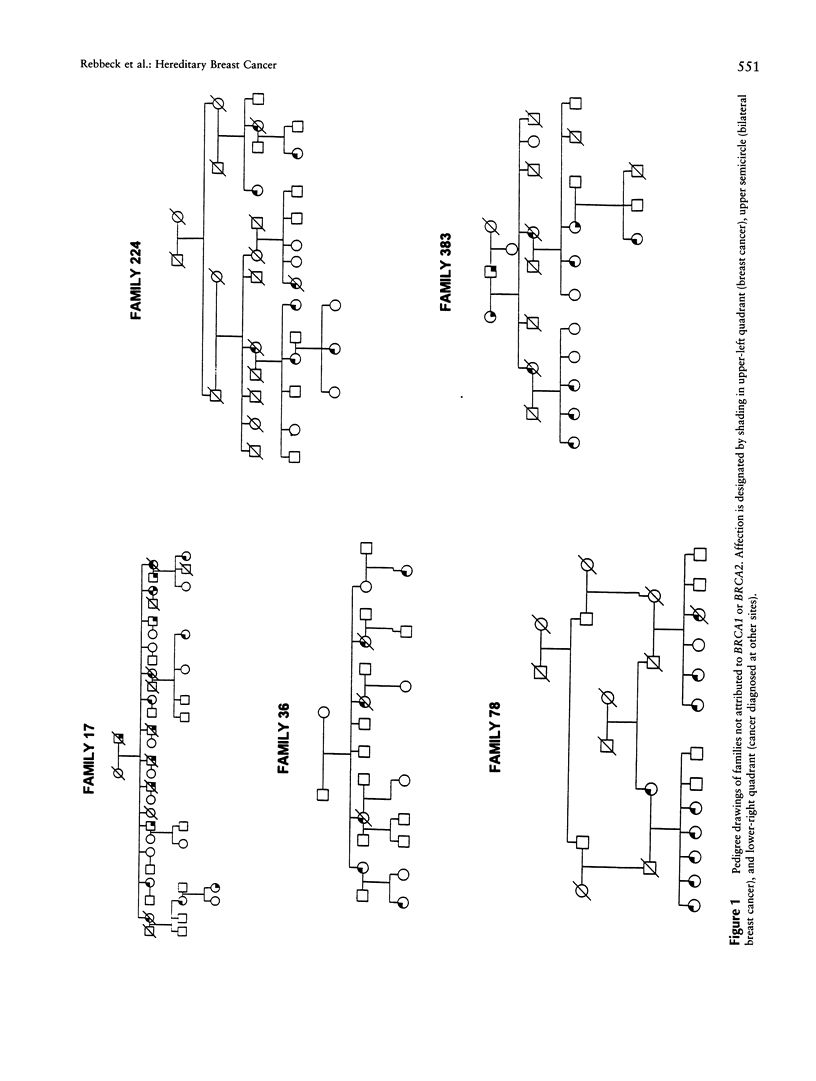
The common hereditary forms of breast cancer have been largely attributed to the inheritance of mutations in the BRCA1 or BRCA2 genes. However, it is not yet clear what proportion of hereditary breast cancer is explained by BRCA1 and BRCA2 or by some other unidentified susceptibility gene(s). We describe the proportion of hereditary breast cancer explained by BRCA1 or BRCA2 in a sample of North American hereditary breast cancers and assess the evidence for additional susceptibility genes that may confer hereditary breast or ovarian cancer risk. Twenty-three families were identified through two high-risk breast cancer research programs. Genetic analysis was undertaken to establish linkage between the breast or ovarian cancer cases and markers on chromosomes 17q (BRCA1) and 13q (BRCA2). Mutation analysis in the BRCA1 and BRCA2 genes was also undertaken in all families. The pattern of hereditary cancer in 14 (61%) of the 23 families studied was attributed to BRCA1 by a combination of linkage and mutation analyses. No families were attributed to BRCA2. Five families (22%) provided evidence against linkage to both BRCA1 and BRCA2. No BRCA1 or BRCA2 mutations were detected in these five families. The BRCA1 or BRCA2 status of four families (17%) could not be determined. BRCA1 and BRCA2 probably explain the majority of hereditary breast cancer that exists in the North American population. However, one or more additional genes may yet be found that explain some proportion of hereditary breast cancer.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buller R. E., Anderson B., Connor J. P., Robinson R. Familial ovarian cancer. Gynecol Oncol. 1993 Nov;51(2):160–166. doi: 10.1006/gyno.1993.1265. [DOI] [PubMed] [Google Scholar]
- Castilla L. H., Couch F. J., Erdos M. R., Hoskins K. F., Calzone K., Garber J. E., Boyd J., Lubin M. B., Deshano M. L., Brody L. C. Mutations in the BRCA1 gene in families with early-onset breast and ovarian cancer. Nat Genet. 1994 Dec;8(4):387–391. doi: 10.1038/ng1294-387. [DOI] [PubMed] [Google Scholar]
- Couch F. J., Farid L. M., DeShano M. L., Tavtigian S. V., Calzone K., Campeau L., Peng Y., Bogden B., Chen Q., Neuhausen S. BRCA2 germline mutations in male breast cancer cases and breast cancer families. Nat Genet. 1996 May;13(1):123–125. doi: 10.1038/ng0596-123. [DOI] [PubMed] [Google Scholar]
- Easton D. F., Bishop D. T., Ford D., Crockford G. P. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1993 Apr;52(4):678–701. [PMC free article] [PubMed] [Google Scholar]
- Ganguly A., Rock M. J., Prockop D. J. Conformation-sensitive gel electrophoresis for rapid detection of single-base differences in double-stranded PCR products and DNA fragments: evidence for solvent-induced bends in DNA heteroduplexes. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10325–10329. doi: 10.1073/pnas.90.21.10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins K. F., Stopfer J. E., Calzone K. A., Merajver S. D., Rebbeck T. R., Garber J. E., Weber B. L. Assessment and counseling for women with a family history of breast cancer. A guide for clinicians. JAMA. 1995 Feb 15;273(7):577–585. [PubMed] [Google Scholar]
- Jolly K. W., Malkin D., Douglass E. C., Brown T. F., Sinclair A. E., Look A. T. Splice-site mutation of the p53 gene in a family with hereditary breast-ovarian cancer. Oncogene. 1994 Jan;9(1):97–102. [PubMed] [Google Scholar]
- Lange K., Weeks D., Boehnke M. Programs for Pedigree Analysis: MENDEL, FISHER, and dGENE. Genet Epidemiol. 1988;5(6):471–472. doi: 10.1002/gepi.1370050611. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narod S. A., Ford D., Devilee P., Barkardottir R. B., Lynch H. T., Smith S. A., Ponder B. A., Weber B. L., Garber J. E., Birch J. M. An evaluation of genetic heterogeneity in 145 breast-ovarian cancer families. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995 Jan;56(1):254–264. [PMC free article] [PubMed] [Google Scholar]
- Phelan C. M., Lancaster J. M., Tonin P., Gumbs C., Cochran C., Carter R., Ghadirian P., Perret C., Moslehi R., Dion F. Mutation analysis of the BRCA2 gene in 49 site-specific breast cancer families. Nat Genet. 1996 May;13(1):120–122. doi: 10.1038/ng0596-120. [DOI] [PubMed] [Google Scholar]
- Ploughman L. M., Boehnke M. Estimating the power of a proposed linkage study for a complex genetic trait. Am J Hum Genet. 1989 Apr;44(4):543–551. [PMC free article] [PubMed] [Google Scholar]
- Savitsky K., Bar-Shira A., Gilad S., Rotman G., Ziv Y., Vanagaite L., Tagle D. A., Smith S., Uziel T., Sfez S. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995 Jun 23;268(5218):1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- Shattuck-Eidens D., McClure M., Simard J., Labrie F., Narod S., Couch F., Hoskins K., Weber B., Castilla L., Erdos M. A collaborative survey of 80 mutations in the BRCA1 breast and ovarian cancer susceptibility gene. Implications for presymptomatic testing and screening. JAMA. 1995 Feb 15;273(7):535–541. [PubMed] [Google Scholar]
- Sobol H., Birnbaum D., Eisinger F. Evidence for a third breast-cancer susceptibility gene. Lancet. 1994 Oct 22;344(8930):1151–1152. doi: 10.1016/s0140-6736(94)90655-6. [DOI] [PubMed] [Google Scholar]
- Wooster R., Neuhausen S. L., Mangion J., Quirk Y., Ford D., Collins N., Nguyen K., Seal S., Tran T., Averill D. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12-13. Science. 1994 Sep 30;265(5181):2088–2090. doi: 10.1126/science.8091231. [DOI] [PubMed] [Google Scholar]


