Abstract
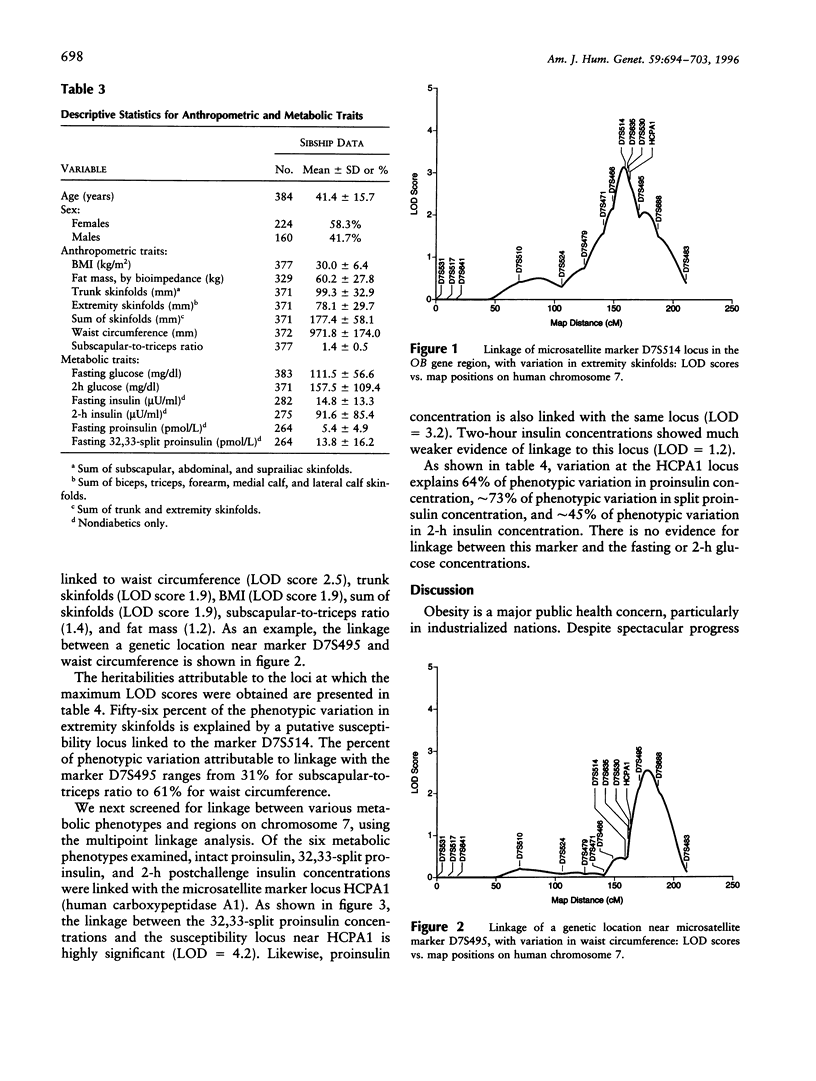
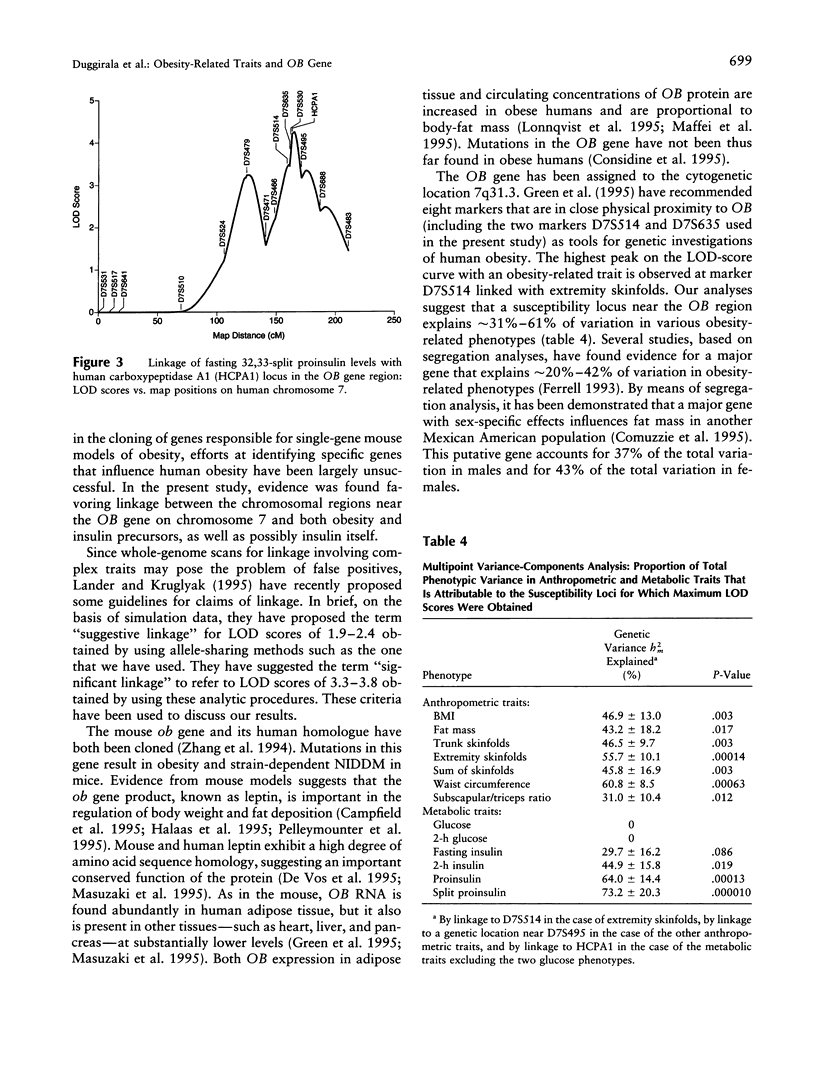
Despite the evidence that human obesity has strong genetic determinants, efforts at identifying specific genes that influence human obesity have largely been unsuccessful. Using the sibship data obtained from 32 low income Mexican American pedigrees ascertained on a type II diabetic proband and a multipoint variance-components method, we tested for linkage between various obesity-related traits plus associated metabolic traits and 15 markers on human chromosome 7. We found evidence for linkage between markers in the OB gene region and various traits, as follows: D7S514 and extremity skinfolds (LOD = 3.1), human carboxypeptidase A1 (HCPA1) and 32,33-split proinsulin level (LOD = 4.2), and HCPA1 and proinsulin level (LOD = 3.2). A putative susceptibility locus linked to the marker D7S514 explained 56% of the total phenotypic variation in extremity skinfolds. Variation at the HCPA1 locus explained 64% of phenotypic variation in proinsulin level and approximately 73% of phenotypic variation in split proinsulin concentration, respectively. Weaker evidence for linkage to several other obesity-related traits (e.g., waist circumference, body-mass index, fat mass by bioimpedance, etc.) was observed for a genetic location, which is approximately 15 cM telomeric to OB. In conclusion, our study reveals that the OB region plays a significant role in determining the phenotypic variation of both insulin precursors and obesity-related traits, at least in Mexican Americans.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos C. I. Robust variance-components approach for assessing genetic linkage in pedigrees. Am J Hum Genet. 1994 Mar;54(3):535–543. [PMC free article] [PubMed] [Google Scholar]
- Beaty T. H., Self S. G., Liang K. Y., Connolly M. A., Chase G. A., Kwiterovich P. O. Use of robust variance components models to analyse triglyceride data in families. Ann Hum Genet. 1985 Oct;49(Pt 4):315–328. doi: 10.1111/j.1469-1809.1985.tb01707.x. [DOI] [PubMed] [Google Scholar]
- Blangero J. Genetic analysis of a common oligogenic trait with quantitative correlates: summary of GAW9 results. Genet Epidemiol. 1995;12(6):689–706. doi: 10.1002/gepi.1370120628. [DOI] [PubMed] [Google Scholar]
- Borecki I. B., Rice T., Pérusse L., Bouchard C., Rao D. C. An exploratory investigation of genetic linkage with body composition and fatness phenotypes: the Québec Family Study. Obes Res. 1994 May;2(3):213–219. doi: 10.1002/j.1550-8528.1994.tb00050.x. [DOI] [PubMed] [Google Scholar]
- Bouchard C. Genetics of obesity: an update on molecular markers. Int J Obes Relat Metab Disord. 1995 Sep;19 (Suppl 3):S10–S13. [PubMed] [Google Scholar]
- Bouchard C., Pérusse L. Heredity and body fat. Annu Rev Nutr. 1988;8:259–277. doi: 10.1146/annurev.nu.08.070188.001355. [DOI] [PubMed] [Google Scholar]
- Burns T. L., Moll P. P., Lauer R. M. Genetic models of human obesity--family studies. Crit Rev Food Sci Nutr. 1993;33(4-5):339–343. doi: 10.1080/10408399309527630. [DOI] [PubMed] [Google Scholar]
- Campfield L. A., Smith F. J., Guisez Y., Devos R., Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995 Jul 28;269(5223):546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- Clement K., Garner C., Hager J., Philippi A., LeDuc C., Carey A., Harris T. J., Jury C., Cardon L. R., Basdevant A. Indication for linkage of the human OB gene region with extreme obesity. Diabetes. 1996 May;45(5):687–690. doi: 10.2337/diab.45.5.687. [DOI] [PubMed] [Google Scholar]
- Comuzzie A. G., Blangero J., Mahaney M. C., Mitchell B. D., Hixson J. E., Samollow P. B., Stern M. P., MacCluer J. W. Major gene with sex-specific effects influences fat mass in Mexican Americans. Genet Epidemiol. 1995;12(5):475–488. doi: 10.1002/gepi.1370120505. [DOI] [PubMed] [Google Scholar]
- Considine R. V., Considine E. L., Williams C. J., Nyce M. R., Magosin S. A., Bauer T. L., Rosato E. L., Colberg J., Caro J. F. Evidence against either a premature stop codon or the absence of obese gene mRNA in human obesity. J Clin Invest. 1995 Jun;95(6):2986–2988. doi: 10.1172/JCI118007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos P., Saladin R., Auwerx J., Staels B. Induction of ob gene expression by corticosteroids is accompanied by body weight loss and reduced food intake. J Biol Chem. 1995 Jul 7;270(27):15958–15961. doi: 10.1074/jbc.270.27.15958. [DOI] [PubMed] [Google Scholar]
- Fasman K. H., Cuticchia A. J., Kingsbury D. T. The GDB Human Genome Data Base anno 1994. Nucleic Acids Res. 1994 Sep;22(17):3462–3469. doi: 10.1093/nar/22.17.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell R. E. Obesity: choosing genetic approaches from a mixed menu. Hum Biol. 1993 Dec;65(6):967–975. [PubMed] [Google Scholar]
- Fulker D. W., Cardon L. R. A sib-pair approach to interval mapping of quantitative trait loci. Am J Hum Genet. 1994 Jun;54(6):1092–1103. [PMC free article] [PubMed] [Google Scholar]
- Fulker D. W., Cherny S. S., Cardon L. R. Multipoint interval mapping of quantitative trait loci, using sib pairs. Am J Hum Genet. 1995 May;56(5):1224–1233. [PMC free article] [PubMed] [Google Scholar]
- Goldgar D. E. Multipoint analysis of human quantitative genetic variation. Am J Hum Genet. 1990 Dec;47(6):957–967. [PMC free article] [PubMed] [Google Scholar]
- Green E. D., Maffei M., Braden V. V., Proenca R., DeSilva U., Zhang Y., Chua S. C., Jr, Leibel R. L., Weissenbach J., Friedman J. M. The human obese (OB) gene: RNA expression pattern and mapping on the physical, cytogenetic, and genetic maps of chromosome 7. Genome Res. 1995 Aug;5(1):5–12. doi: 10.1101/gr.5.1.5. [DOI] [PubMed] [Google Scholar]
- Gyapay G., Morissette J., Vignal A., Dib C., Fizames C., Millasseau P., Marc S., Bernardi G., Lathrop M., Weissenbach J. The 1993-94 Généthon human genetic linkage map. Nat Genet. 1994 Jun;7(2 Spec No):246–339. doi: 10.1038/ng0694supp-246. [DOI] [PubMed] [Google Scholar]
- Haffner S. M., Stern M. P., Hazuda H. P., Pugh J., Patterson J. K. Do upper-body and centralized adiposity measure different aspects of regional body-fat distribution? Relationship to non-insulin-dependent diabetes mellitus, lipids, and lipoproteins. Diabetes. 1987 Jan;36(1):43–51. doi: 10.2337/diab.36.1.43. [DOI] [PubMed] [Google Scholar]
- Halaas J. L., Gajiwala K. S., Maffei M., Cohen S. L., Chait B. T., Rabinowitz D., Lallone R. L., Burley S. K., Friedman J. M. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995 Jul 28;269(5223):543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Haseman J. K., Elston R. C. The investigation of linkage between a quantitative trait and a marker locus. Behav Genet. 1972 Mar;2(1):3–19. doi: 10.1007/BF01066731. [DOI] [PubMed] [Google Scholar]
- Hopper J. L., Mathews J. D. Extensions to multivariate normal models for pedigree analysis. Ann Hum Genet. 1982 Oct;46(Pt 4):373–383. doi: 10.1111/j.1469-1809.1982.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Kahn S. E., Leonetti D. L., Prigeon R. L., Boyko E. J., Bergstrom R. W., Fujimoto W. Y. Proinsulin as a marker for the development of NIDDM in Japanese-American men. Diabetes. 1995 Feb;44(2):173–179. doi: 10.2337/diab.44.2.173. [DOI] [PubMed] [Google Scholar]
- Lander E., Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet. 1995 Nov;11(3):241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- Lange K., Weeks D., Boehnke M. Programs for Pedigree Analysis: MENDEL, FISHER, and dGENE. Genet Epidemiol. 1988;5(6):471–472. doi: 10.1002/gepi.1370050611. [DOI] [PubMed] [Google Scholar]
- Lee S., Zambas E. D., Marsh W. L., Redman C. M. The human Kell blood group gene maps to chromosome 7q33 and its expression is restricted to erythroid cells. Blood. 1993 May 15;81(10):2804–2809. [PubMed] [Google Scholar]
- Leibel R. L., Bahary N., Friedman J. M. Strategies for the molecular genetic analysis of obesity in humans. Crit Rev Food Sci Nutr. 1993;33(4-5):351–358. doi: 10.1080/10408399309527632. [DOI] [PubMed] [Google Scholar]
- Ludvik B., Nolan J. J., Baloga J., Sacks D., Olefsky J. Effect of obesity on insulin resistance in normal subjects and patients with NIDDM. Diabetes. 1995 Sep;44(9):1121–1125. doi: 10.2337/diab.44.9.1121. [DOI] [PubMed] [Google Scholar]
- Lönnqvist F., Arner P., Nordfors L., Schalling M. Overexpression of the obese (ob) gene in adipose tissue of human obese subjects. Nat Med. 1995 Sep;1(9):950–953. doi: 10.1038/nm0995-950. [DOI] [PubMed] [Google Scholar]
- Maffei M., Halaas J., Ravussin E., Pratley R. E., Lee G. H., Zhang Y., Fei H., Kim S., Lallone R., Ranganathan S. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995 Nov;1(11):1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- Malide D., Seidah N. G., Chrétien M., Bendayan M. Electron microscopic immunocytochemical evidence for the involvement of the convertases PC1 and PC2 in the processing of proinsulin in pancreatic beta-cells. J Histochem Cytochem. 1995 Jan;43(1):11–19. doi: 10.1177/43.1.7822759. [DOI] [PubMed] [Google Scholar]
- Manser E., Fernandez D., Loo L., Goh P. Y., Monfries C., Hall C., Lim L. Human carboxypeptidase E. Isolation and characterization of the cDNA, sequence conservation, expression and processing in vitro. Biochem J. 1990 Apr 15;267(2):517–525. doi: 10.1042/bj2670517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuzaki H., Ogawa Y., Isse N., Satoh N., Okazaki T., Shigemoto M., Mori K., Tamura N., Hosoda K., Yoshimasa Y. Human obese gene expression. Adipocyte-specific expression and regional differences in the adipose tissue. Diabetes. 1995 Jul;44(7):855–858. doi: 10.2337/diab.44.7.855. [DOI] [PubMed] [Google Scholar]
- Naggert J. K., Fricker L. D., Varlamov O., Nishina P. M., Rouille Y., Steiner D. F., Carroll R. J., Paigen B. J., Leiter E. H. Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nat Genet. 1995 Jun;10(2):135–142. doi: 10.1038/ng0695-135. [DOI] [PubMed] [Google Scholar]
- Oohashi H., Ohgawara H., Nanjo K., Tasaka Y., Cao Q. P., Chan S. J., Rubenstein A. H., Steiner D. F., Omori Y. Familial hyperproinsulinemia associated with NIDDM. A case study. Diabetes Care. 1993 Oct;16(10):1340–1346. doi: 10.2337/diacare.16.10.1340. [DOI] [PubMed] [Google Scholar]
- Pelleymounter M. A., Cullen M. J., Baker M. B., Hecht R., Winters D., Boone T., Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995 Jul 28;269(5223):540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- Perriello G., Misericordia P., Volpi E., Pampanelli S., Santeusanio F., Brunetti P., Bolli G. B. Contribution of obesity to insulin resistance in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1995 Aug;80(8):2464–2469. doi: 10.1210/jcem.80.8.7629243. [DOI] [PubMed] [Google Scholar]
- Phillips D. I., Clark P. M., Hales C. N., Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994 Apr;11(3):286–292. doi: 10.1111/j.1464-5491.1994.tb00273.x. [DOI] [PubMed] [Google Scholar]
- Reed D. R., Ding Y., Xu W., Cather C., Green E. D., Price R. A. Extreme obesity may be linked to markers flanking the human OB gene. Diabetes. 1996 May;45(5):691–694. doi: 10.2337/diab.45.5.691. [DOI] [PubMed] [Google Scholar]
- Reed D. R., Ding Y., Xu W., Cather C., Price R. A. Human obesity does not segregate with the chromosomal regions of Prader-Willi, Bardet-Biedl, Cohen, Borjeson or Wilson-Turner syndromes. Int J Obes Relat Metab Disord. 1995 Sep;19(9):599–603. [PubMed] [Google Scholar]
- Rhodes C. J., Alarcón C. What beta-cell defect could lead to hyperproinsulinemia in NIDDM? Some clues from recent advances made in understanding the proinsulin-processing mechanism. Diabetes. 1994 Apr;43(4):511–517. doi: 10.2337/diab.43.4.511. [DOI] [PubMed] [Google Scholar]
- Rice T., Borecki I. B., Bouchard C., Rao D. C. Segregation analysis of fat mass and other body composition measures derived from underwater weighing. Am J Hum Genet. 1993 May;52(5):967–973. [PMC free article] [PubMed] [Google Scholar]
- Saad M. F., Kahn S. E., Nelson R. G., Pettitt D. J., Knowler W. C., Schwartz M. W., Kowalyk S., Bennett P. H., Porte D., Jr Disproportionately elevated proinsulin in Pima Indians with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1990 May;70(5):1247–1253. doi: 10.1210/jcem-70-5-1247. [DOI] [PubMed] [Google Scholar]
- Schork N. J. Extended multipoint identity-by-descent analysis of human quantitative traits: efficiency, power, and modeling considerations. Am J Hum Genet. 1993 Dec;53(6):1306–1319. [PMC free article] [PubMed] [Google Scholar]
- Seidah N. G., Chrétien M. Pro-protein convertases of subtilisin/kexin family. Methods Enzymol. 1994;244:175–188. doi: 10.1016/0076-6879(94)44015-8. [DOI] [PubMed] [Google Scholar]
- Sobey W. J., Beer S. F., Carrington C. A., Clark P. M., Frank B. H., Gray I. P., Luzio S. D., Owens D. R., Schneider A. E., Siddle K. Sensitive and specific two-site immunoradiometric assays for human insulin, proinsulin, 65-66 split and 32-33 split proinsulins. Biochem J. 1989 Jun 1;260(2):535–541. doi: 10.1042/bj2600535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M. P., Haffner S. M. Type II diabetes and its complications in Mexican Americans. Diabetes Metab Rev. 1990 Feb;6(1):29–45. doi: 10.1002/dmr.5610060102. [DOI] [PubMed] [Google Scholar]
- Stirling B., Cox N. J., Bell G. I., Hanis C. L., Spielman R. S., Concannon P. Identification of microsatellite markers near the human ob gene and linkage studies in NIDDM-affected sib pairs. Diabetes. 1995 Aug;44(8):999–1001. doi: 10.2337/diab.44.8.999. [DOI] [PubMed] [Google Scholar]
- Temple R. C., Clark P. M., Nagi D. K., Schneider A. E., Yudkin J. S., Hales C. N. Radioimmunoassay may overestimate insulin in non-insulin-dependent diabetics. Clin Endocrinol (Oxf) 1990 Jun;32(6):689–693. doi: 10.1111/j.1365-2265.1990.tb00915.x. [DOI] [PubMed] [Google Scholar]
- Tsui L. C., Donis-Keller H., Grzeschik K. H. Report of the second international workshop on human chromosome 7 mapping 1994. Cytogenet Cell Genet. 1995;71(1):2–21. [PubMed] [Google Scholar]
- Walker M. Obesity, insulin resistance, and its link to non-insulin-dependent diabetes mellitus. Metabolism. 1995 Sep;44(9 Suppl 3):18–20. doi: 10.1016/0026-0495(95)90314-3. [DOI] [PubMed] [Google Scholar]
- Warden C. H., Fisler J. S., Shoemaker S. M., Wen P. Z., Svenson K. L., Pace M. J., Lusis A. J. Identification of four chromosomal loci determining obesity in a multifactorial mouse model. J Clin Invest. 1995 Apr;95(4):1545–1552. doi: 10.1172/JCI117827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Ohagi S., Sanke T., Furuta H., Furuta M., Nanjo K. Association of the prohormone convertase 2 gene (PCSK2) on chromosome 20 with NIDDM in Japanese subjects. Diabetes. 1995 Apr;44(4):389–393. doi: 10.2337/diab.44.4.389. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J. M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994 Dec 1;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]


