Abstract
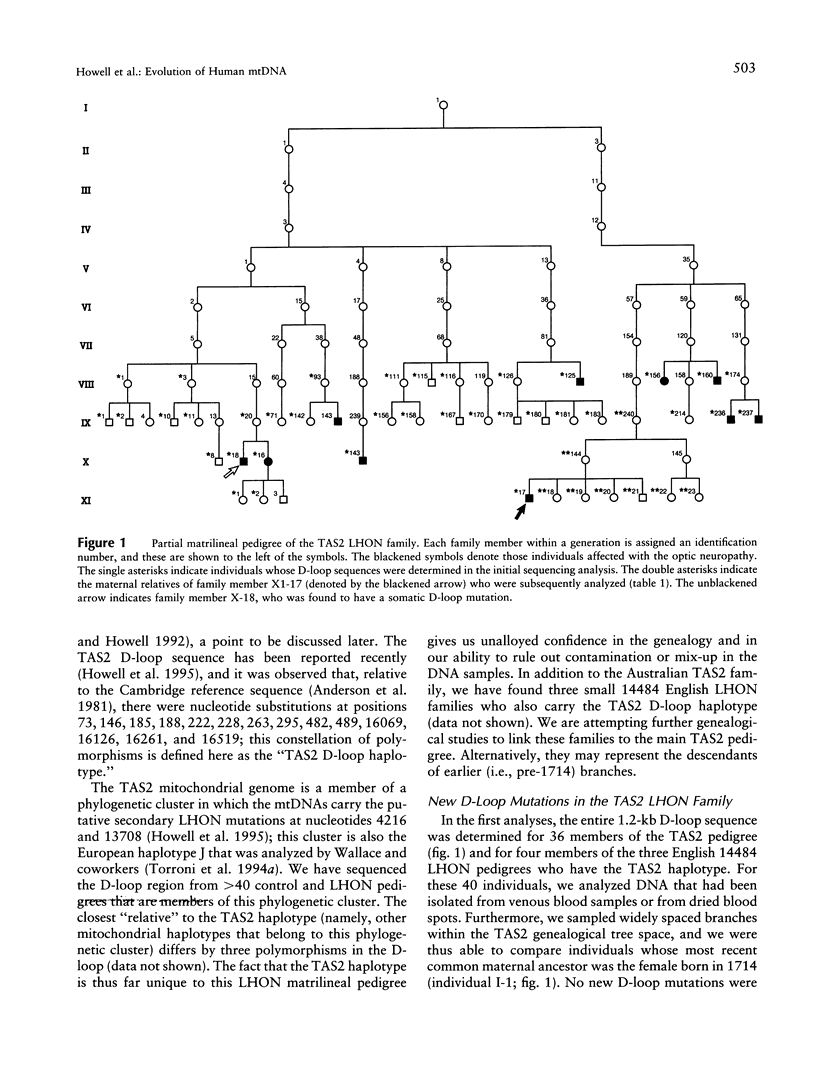
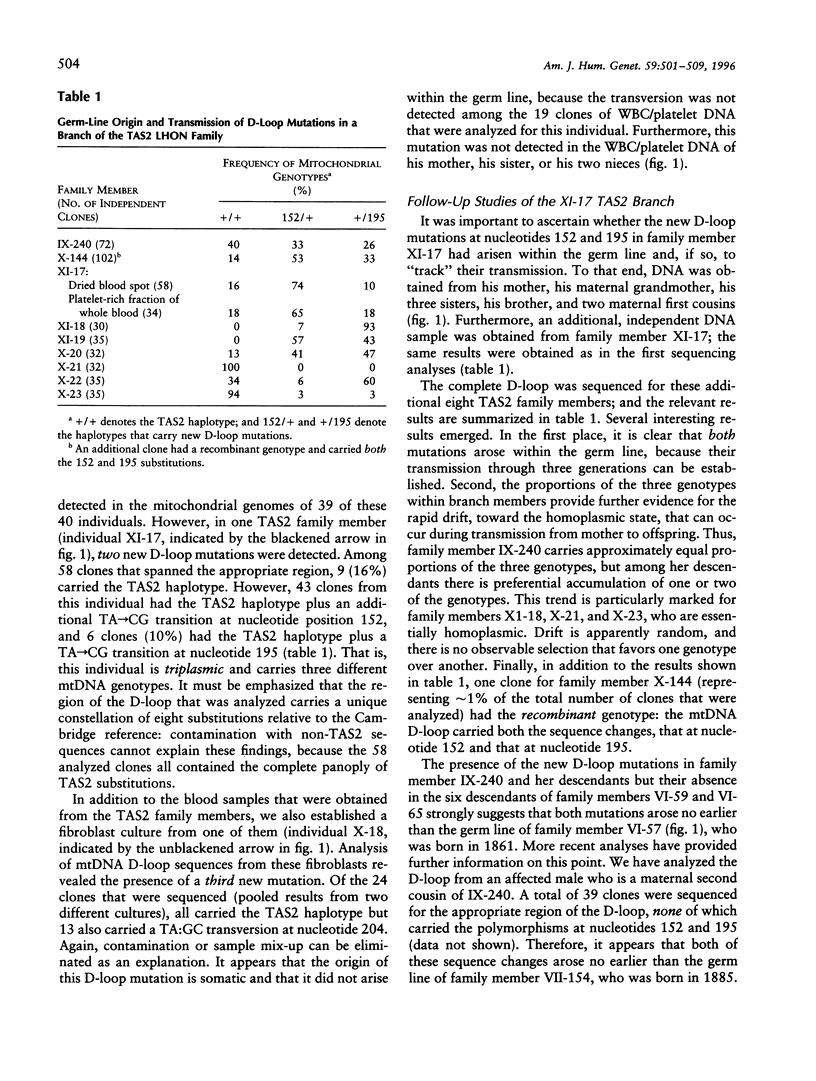
The results of an empirical nucleotide-sequencing approach indicate that the evolution of the human mitochondrial noncoding D-loop is both more rapid and more complex than is revealed by standard phylogenetic approaches. The nucleotide sequence of the D-loop region of the mitochondrial genome was determined for 45 members of a large matrilineal Leber hereditary optic neuropathy pedigree. Two germ-line mutations have arisen in members of one branch of the family, thereby leading to triplasmic descendants with three mitochondrial genotypes. Segregation toward the homoplasmic state can occur within a single generation in some of these descendants, a result that suggests rapid fixation of mitochondrial mutations as a result of developmental bottlenecking. However, slow segregation was observed in other offspring, and therefore no single or simple pattern of segregation can be generalized from the available data. Evidence for rare mtDNA recombination within the D-loop was obtained for one family member. In addition to these germ-line mutations, a somatic mutation was found in the D-loop of one family member. When this genealogical approach was applied to the nucleotide sequences of mitochondrial coding regions, the results again indicated a very rapid rate of evolution.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Armour J. A., Anttinen T., May C. A., Vega E. E., Sajantila A., Kidd J. R., Kidd K. K., Bertranpetit J., Päbo S., Jeffreys A. J. Minisatellite diversity supports a recent African origin for modern humans. Nat Genet. 1996 Jun;13(2):154–160. doi: 10.1038/ng0696-154. [DOI] [PubMed] [Google Scholar]
- Ballard J. W., Kreitman M. Unraveling selection in the mitochondrial genome of Drosophila. Genetics. 1994 Nov;138(3):757–772. doi: 10.1093/genetics/138.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann R. L., Stoneking M., Wilson A. C. Mitochondrial DNA and human evolution. Nature. 1987 Jan 1;325(6099):31–36. doi: 10.1038/325031a0. [DOI] [PubMed] [Google Scholar]
- Comas D., Päbo S., Bertranpetit J. Heteroplasmy in the control region of human mitochondrial DNA. Genome Res. 1995 Aug;5(1):89–90. doi: 10.1101/gr.5.1.89. [DOI] [PubMed] [Google Scholar]
- Cummings M. P., Otto S. P., Wakeley J. Sampling properties of DNA sequence data in phylogenetic analysis. Mol Biol Evol. 1995 Sep;12(5):814–822. doi: 10.1093/oxfordjournals.molbev.a040258. [DOI] [PubMed] [Google Scholar]
- Donnelly P., Tavaré S. Coalescents and genealogical structure under neutrality. Annu Rev Genet. 1995;29:401–421. doi: 10.1146/annurev.ge.29.120195.002153. [DOI] [PubMed] [Google Scholar]
- Gadaleta G., D'Elia D., Capaccio L., Saccone C., Pepe G. Isolation of a 25-kDa protein binding to a curved DNA upstream the origin of the L strand replication in the rat mitochondrial genome. J Biol Chem. 1996 Jun 7;271(23):13537–13541. doi: 10.1074/jbc.271.23.13537. [DOI] [PubMed] [Google Scholar]
- Ghosh S. S., Fahy E., Bodis-Wollner I., Sherman J., Howell N. Longitudinal study of a heteroplasmic 3460 Leber hereditary optic neuropathy family by multiplexed primer-extension analysis and nucleotide sequencing. Am J Hum Genet. 1996 Feb;58(2):325–334. [PMC free article] [PubMed] [Google Scholar]
- Gill P., Ivanov P. L., Kimpton C., Piercy R., Benson N., Tully G., Evett I., Hagelberg E., Sullivan K. Identification of the remains of the Romanov family by DNA analysis. Nat Genet. 1994 Feb;6(2):130–135. doi: 10.1038/ng0294-130. [DOI] [PubMed] [Google Scholar]
- Hammer M. F. A recent common ancestry for human Y chromosomes. Nature. 1995 Nov 23;378(6555):376–378. doi: 10.1038/378376a0. [DOI] [PubMed] [Google Scholar]
- Hauswirth W. W., Laipis P. J. Mitochondrial DNA polymorphism in a maternal lineage of Holstein cows. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4686–4690. doi: 10.1073/pnas.79.15.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horai S., Hayasaka K., Kondo R., Tsugane K., Takahata N. Recent African origin of modern humans revealed by complete sequences of hominoid mitochondrial DNAs. Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):532–536. doi: 10.1073/pnas.92.2.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell N., Bindoff L. A., McCullough D. A., Kubacka I., Poulton J., Mackey D., Taylor L., Turnbull D. M. Leber hereditary optic neuropathy: identification of the same mitochondrial ND1 mutation in six pedigrees. Am J Hum Genet. 1991 Nov;49(5):939–950. [PMC free article] [PubMed] [Google Scholar]
- Howell N., Halvorson S., Kubacka I., McCullough D. A., Bindoff L. A., Turnbull D. M. Mitochondrial gene segregation in mammals: is the bottleneck always narrow? Hum Genet. 1992 Sep-Oct;90(1-2):117–120. doi: 10.1007/BF00210753. [DOI] [PubMed] [Google Scholar]
- Howell N., Kubacka I., Halvorson S., Howell B., McCullough D. A., Mackey D. Phylogenetic analysis of the mitochondrial genomes from Leber hereditary optic neuropathy pedigrees. Genetics. 1995 May;140(1):285–302. doi: 10.1093/genetics/140.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell N., Kubacka I., Xu M., McCullough D. A. Leber hereditary optic neuropathy: involvement of the mitochondrial ND1 gene and evidence for an intragenic suppressor mutation. Am J Hum Genet. 1991 May;48(5):935–942. [PMC free article] [PubMed] [Google Scholar]
- Ivanov P. L., Wadhams M. J., Roby R. K., Holland M. M., Weedn V. W., Parsons T. J. Mitochondrial DNA sequence heteroplasmy in the Grand Duke of Russia Georgij Romanov establishes the authenticity of the remains of Tsar Nicholas II. Nat Genet. 1996 Apr;12(4):417–420. doi: 10.1038/ng0496-417. [DOI] [PubMed] [Google Scholar]
- Lundstrom R., Tavaré S., Ward R. H. Estimating substitution rates from molecular data using the coalescent. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5961–5965. doi: 10.1073/pnas.89.13.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey D. A., Buttery R. G. Leber hereditary optic neuropathy in Australia. Aust N Z J Ophthalmol. 1992 Aug;20(3):177–184. doi: 10.1111/j.1442-9071.1992.tb00937.x. [DOI] [PubMed] [Google Scholar]
- Mackey D., Howell N. A variant of Leber hereditary optic neuropathy characterized by recovery of vision and by an unusual mitochondrial genetic etiology. Am J Hum Genet. 1992 Dec;51(6):1218–1228. [PMC free article] [PubMed] [Google Scholar]
- Nachman M. W., Brown W. M., Stoneking M., Aquadro C. F. Nonneutral mitochondrial DNA variation in humans and chimpanzees. Genetics. 1996 Mar;142(3):953–963. doi: 10.1093/genetics/142.3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny D., Steel M., Waddell P. J., Hendy M. D. Improved analyses of human mtDNA sequences support a recent African origin for Homo sapiens. Mol Biol Evol. 1995 Sep;12(5):863–882. doi: 10.1093/oxfordjournals.molbev.a040263. [DOI] [PubMed] [Google Scholar]
- Pesole G., Sbisá E., Preparata G., Saccone C. The evolution of the mitochondrial D-loop region and the origin of modern man. Mol Biol Evol. 1992 Jul;9(4):587–598. doi: 10.1093/oxfordjournals.molbev.a040747. [DOI] [PubMed] [Google Scholar]
- Päbo S., Irwin D. M., Wilson A. C. DNA damage promotes jumping between templates during enzymatic amplification. J Biol Chem. 1990 Mar 15;265(8):4718–4721. [PubMed] [Google Scholar]
- Rand D. M., Dorfsman M., Kann L. M. Neutral and non-neutral evolution of Drosophila mitochondrial DNA. Genetics. 1994 Nov;138(3):741–756. doi: 10.1093/genetics/138.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvolo M. A new approach to studying modern human origins: hypothesis testing with coalescence time distributions. Mol Phylogenet Evol. 1996 Feb;5(1):202–219. doi: 10.1006/mpev.1996.0014. [DOI] [PubMed] [Google Scholar]
- Sherry S. T., Rogers A. R., Harpending H., Soodyall H., Jenkins T., Stoneking M. Mismatch distributions of mtDNA reveal recent human population expansions. Hum Biol. 1994 Oct;66(5):761–775. [PubMed] [Google Scholar]
- Stoneking M., Sherry S. T., Redd A. J., Vigilant L. New approaches to dating suggest a recent age for the human mtDNA ancestor. Philos Trans R Soc Lond B Biol Sci. 1992 Aug 29;337(1280):167–175. doi: 10.1098/rstb.1992.0094. [DOI] [PubMed] [Google Scholar]
- Tamura K., Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993 May;10(3):512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Tishkoff S. A., Dietzsch E., Speed W., Pakstis A. J., Kidd J. R., Cheung K., Bonné-Tamir B., Santachiara-Benerecetti A. S., Moral P., Krings M. Global patterns of linkage disequilibrium at the CD4 locus and modern human origins. Science. 1996 Mar 8;271(5254):1380–1387. doi: 10.1126/science.271.5254.1380. [DOI] [PubMed] [Google Scholar]
- Torroni A., Lott M. T., Cabell M. F., Chen Y. S., Lavergne L., Wallace D. C. mtDNA and the origin of Caucasians: identification of ancient Caucasian-specific haplogroups, one of which is prone to a recurrent somatic duplication in the D-loop region. Am J Hum Genet. 1994 Oct;55(4):760–776. [PMC free article] [PubMed] [Google Scholar]
- Torroni A., Neel J. V., Barrantes R., Schurr T. G., Wallace D. C. Mitochondrial DNA "clock" for the Amerinds and its implications for timing their entry into North America. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1158–1162. doi: 10.1073/pnas.91.3.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigilant L., Stoneking M., Harpending H., Hawkes K., Wilson A. C. African populations and the evolution of human mitochondrial DNA. Science. 1991 Sep 27;253(5027):1503–1507. doi: 10.1126/science.1840702. [DOI] [PubMed] [Google Scholar]
- Wakeley J. Substitution rate variation among sites in hypervariable region 1 of human mitochondrial DNA. J Mol Evol. 1993 Dec;37(6):613–623. doi: 10.1007/BF00182747. [DOI] [PubMed] [Google Scholar]
- Ward R. H., Frazier B. L., Dew-Jager K., Päbo S. Extensive mitochondrial diversity within a single Amerindian tribe. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8720–8724. doi: 10.1073/pnas.88.19.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield L. S., Sulston J. E., Goodfellow P. N. Sequence variation of the human Y chromosome. Nature. 1995 Nov 23;378(6555):379–380. doi: 10.1038/378379a0. [DOI] [PubMed] [Google Scholar]
- Yang Z. A space-time process model for the evolution of DNA sequences. Genetics. 1995 Feb;139(2):993–1005. doi: 10.1093/genetics/139.2.993. [DOI] [PMC free article] [PubMed] [Google Scholar]



