Abstract
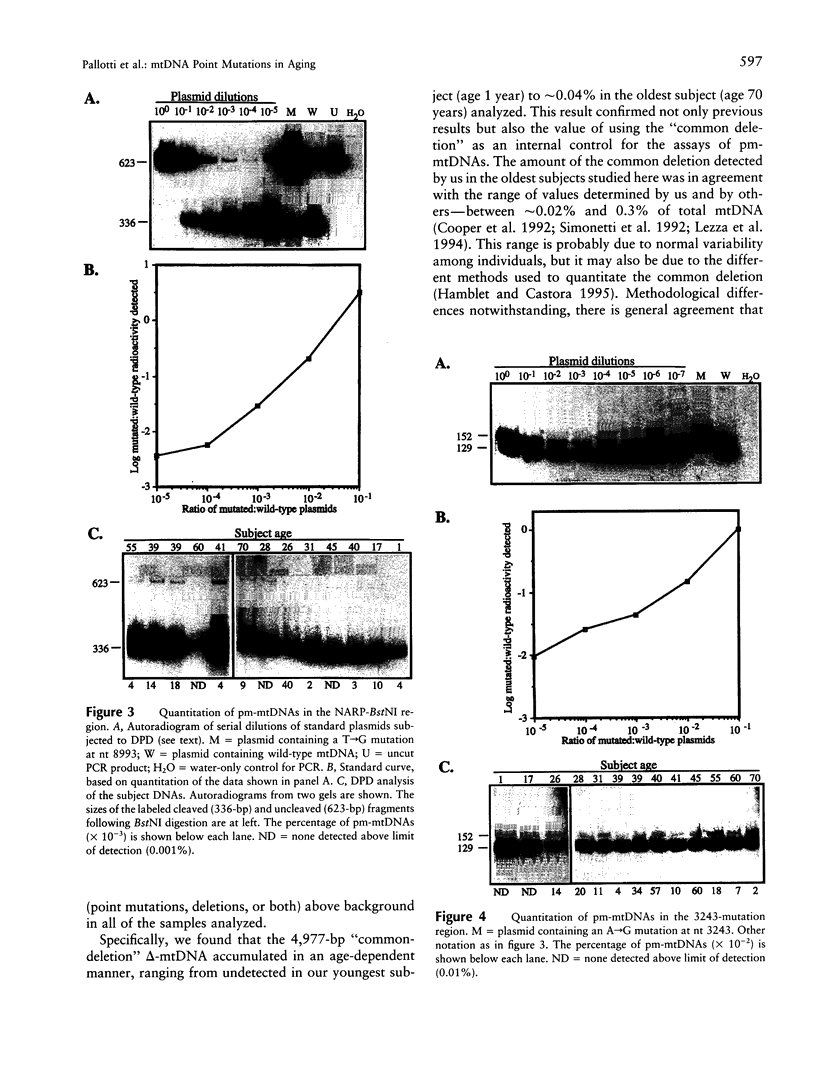
It is unclear at present whether specific mtDNA point mutations accumulate during normal human aging. In order to address this question, we used quantitative PCR of total DNA isolated from skeletal muscle from normal individuals of various ages to search for the presence and amount of spontaneous mtDNA point mutations in two small regions of the human mitochondrial genome. We observed low levels of somatic mutations above background in both regions, but there was no correlation between the amount of mutation detected and the age of the subject. These results contrasted with our finding of an age-related increase in the amount of the mtDNA "common deletion" in these very samples. Thus, it appears that both somatic mtDNA point mutations and mtDNA deletions can arise at low frequency in normal individuals but that, unlike deletions, there is no preferential amplification or accumulation of specific point mutations in skeletal muscle over the course of the normal human life span.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Bindoff L. A., Howell N., Poulton J., McCullough D. A., Morten K. J., Lightowlers R. N., Turnbull D. M., Weber K. Abnormal RNA processing associated with a novel tRNA mutation in mitochondrial DNA. A potential disease mechanism. J Biol Chem. 1993 Sep 15;268(26):19559–19564. [PubMed] [Google Scholar]
- Bodenteich A., Mitchell L. G., Merril C. R. A lifetime of retinal light exposure does not appear to increase mitochondrial mutations. Gene. 1991 Dec 15;108(2):305–309. doi: 10.1016/0378-1119(91)90451-g. [DOI] [PubMed] [Google Scholar]
- Cavelier L., Jazin E. E., Eriksson I., Prince J., Båve U., Oreland L., Gyllensten U. Decreased cytochrome-c oxidase activity and lack of age-related accumulation of mitochondrial DNA deletions in the brains of schizophrenics. Genomics. 1995 Sep 1;29(1):217–224. doi: 10.1006/geno.1995.1234. [DOI] [PubMed] [Google Scholar]
- Chen X., Prosser R., Simonetti S., Sadlock J., Jagiello G., Schon E. A. Rearranged mitochondrial genomes are present in human oocytes. Am J Hum Genet. 1995 Aug;57(2):239–247. [PMC free article] [PubMed] [Google Scholar]
- Clayton D. A., Doda J. N., Friedberg E. C. The absence of a pyrimidine dimer repair mechanism in mammalian mitochondria. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2777–2781. doi: 10.1073/pnas.71.7.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E. Replication of nuclear and mitochondrial DNA in X-ray-damaged cells: evidence for a nuclear-specific mechanism that down-regulates replication. Radiat Res. 1992 Sep;131(3):338–344. [PubMed] [Google Scholar]
- Cooper J. M., Mann V. M., Schapira A. H. Analyses of mitochondrial respiratory chain function and mitochondrial DNA deletion in human skeletal muscle: effect of ageing. J Neurol Sci. 1992 Nov;113(1):91–98. doi: 10.1016/0022-510x(92)90270-u. [DOI] [PubMed] [Google Scholar]
- Corral-Debrinski M., Horton T., Lott M. T., Shoffner J. M., Beal M. F., Wallace D. C. Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat Genet. 1992 Dec;2(4):324–329. doi: 10.1038/ng1292-324. [DOI] [PubMed] [Google Scholar]
- Corral-Debrinski M., Stepien G., Shoffner J. M., Lott M. T., Kanter K., Wallace D. C. Hypoxemia is associated with mitochondrial DNA damage and gene induction. Implications for cardiac disease. JAMA. 1991 Oct 2;266(13):1812–1816. [PubMed] [Google Scholar]
- Cortopassi G. A., Arnheim N. Detection of a specific mitochondrial DNA deletion in tissues of older humans. Nucleic Acids Res. 1990 Dec 11;18(23):6927–6933. doi: 10.1093/nar/18.23.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortopassi G. A., Shibata D., Soong N. W., Arnheim N. A pattern of accumulation of a somatic deletion of mitochondrial DNA in aging human tissues. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7370–7374. doi: 10.1073/pnas.89.16.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croizat B., Attardi G. Selective in vivo damage by "visible" light of BrdU-containing mitochondrial DNA in a thymidine kinase-deficient mouse cell line with persistent mitochondrial enzyme activity. J Cell Sci. 1975 Oct;19(1):69–84. doi: 10.1242/jcs.19.1.69. [DOI] [PubMed] [Google Scholar]
- Driggers W. J., LeDoux S. P., Wilson G. L. Repair of oxidative damage within the mitochondrial DNA of RINr 38 cells. J Biol Chem. 1993 Oct 15;268(29):22042–22045. [PubMed] [Google Scholar]
- Eckert K. A., Kunkel T. A. DNA polymerase fidelity and the polymerase chain reaction. PCR Methods Appl. 1991 Aug;1(1):17–24. doi: 10.1101/gr.1.1.17. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Felley-Bosco E., Pourzand C., Zijlstra J., Amstad P., Cerutti P. A genotypic mutation system measuring mutations in restriction recognition sequences. Nucleic Acids Res. 1991 Jun 11;19(11):2913–2919. doi: 10.1093/nar/19.11.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschenson M., Low R. L., Loehr J. Levels of the mitochondrial endonuclease during rat cardiac development implicate a role for the enzyme in repair of oxidative damage in mitochondrial DNA. J Mol Cell Cardiol. 1994 Jan;26(1):31–40. doi: 10.1006/jmcc.1994.1005. [DOI] [PubMed] [Google Scholar]
- Goto Y., Nonaka I., Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990 Dec 13;348(6302):651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- Hamblet N. S., Castora F. J. Mitochondrial DNA deletion analysis: a comparison of PCR quantitative methods. Biochem Biophys Res Commun. 1995 Feb 15;207(2):839–847. doi: 10.1006/bbrc.1995.1262. [DOI] [PubMed] [Google Scholar]
- Hare J. F., Ching E., Attardi G. Isolation, subunit composition, and site of synthesis of human cytochrome c oxidase. Biochemistry. 1980 May 13;19(10):2023–2030. doi: 10.1021/bi00551a003. [DOI] [PubMed] [Google Scholar]
- Hattori K., Tanaka M., Sugiyama S., Obayashi T., Ito T., Satake T., Hanaki Y., Asai J., Nagano M., Ozawa T. Age-dependent increase in deleted mitochondrial DNA in the human heart: possible contributory factor to presbycardia. Am Heart J. 1991 Jun;121(6 Pt 1):1735–1742. doi: 10.1016/0002-8703(91)90020-i. [DOI] [PubMed] [Google Scholar]
- Hayashi J., Ohta S., Kagawa Y., Kondo H., Kaneda H., Yonekawa H., Takai D., Miyabayashi S. Nuclear but not mitochondrial genome involvement in human age-related mitochondrial dysfunction. Functional integrity of mitochondrial DNA from aged subjects. J Biol Chem. 1994 Mar 4;269(9):6878–6883. [PubMed] [Google Scholar]
- Hegler J., Bittner D., Boiteux S., Epe B. Quantification of oxidative DNA modifications in mitochondria. Carcinogenesis. 1993 Nov;14(11):2309–2312. doi: 10.1093/carcin/14.11.2309. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Petty R. K., Morgan-Hughes J. A. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am J Hum Genet. 1990 Mar;46(3):428–433. [PMC free article] [PubMed] [Google Scholar]
- Kahn S. M., Jiang W., Culbertson T. A., Weinstein I. B., Williams G. M., Tomita N., Ronai Z. Rapid and sensitive nonradioactive detection of mutant K-ras genes via 'enriched' PCR amplification. Oncogene. 1991 Jun;6(6):1079–1083. [PubMed] [Google Scholar]
- Kalinowski D. P., Illenye S., Van Houten B. Analysis of DNA damage and repair in murine leukemia L1210 cells using a quantitative polymerase chain reaction assay. Nucleic Acids Res. 1992 Jul 11;20(13):3485–3494. doi: 10.1093/nar/20.13.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keohavong P., Thilly W. G. Fidelity of DNA polymerases in DNA amplification. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9253–9257. doi: 10.1073/pnas.86.23.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. P., Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science. 1989 Oct 27;246(4929):500–503. doi: 10.1126/science.2814477. [DOI] [PubMed] [Google Scholar]
- Kumar R., Barbacid M. Oncogene detection at the single cell level. Oncogene. 1988 Dec;3(6):647–651. [PubMed] [Google Scholar]
- Lansman R. A., Clayton D. A. Selective nicking of mammalian mitochondrial DNA in vivo: photosensitization by incorporation of 5-bromodeoxyuridine. J Mol Biol. 1975 Dec 25;99(4):761–776. doi: 10.1016/s0022-2836(75)80183-5. [DOI] [PubMed] [Google Scholar]
- LeDoux S. P., Wilson G. L., Beecham E. J., Stevnsner T., Wassermann K., Bohr V. A. Repair of mitochondrial DNA after various types of DNA damage in Chinese hamster ovary cells. Carcinogenesis. 1992 Nov;13(11):1967–1973. doi: 10.1093/carcin/13.11.1967. [DOI] [PubMed] [Google Scholar]
- Lezza A. M., Boffoli D., Scacco S., Cantatore P., Gadaleta M. N. Correlation between mitochondrial DNA 4977-bp deletion and respiratory chain enzyme activities in aging human skeletal muscles. Biochem Biophys Res Commun. 1994 Nov 30;205(1):772–779. doi: 10.1006/bbrc.1994.2732. [DOI] [PubMed] [Google Scholar]
- Ling L. L., Keohavong P., Dias C., Thilly W. G. Optimization of the polymerase chain reaction with regard to fidelity: modified T7, Taq, and vent DNA polymerases. PCR Methods Appl. 1991 Aug;1(1):63–69. doi: 10.1101/gr.1.1.63. [DOI] [PubMed] [Google Scholar]
- Melov S., Shoffner J. M., Kaufman A., Wallace D. C. Marked increase in the number and variety of mitochondrial DNA rearrangements in aging human skeletal muscle. Nucleic Acids Res. 1995 Oct 25;23(20):4122–4126. doi: 10.1093/nar/23.20.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita S., Monnat R. J., Jr, Loeb L. A. Resistance of HeLa cell mitochondrial DNA to mutagenesis by chemical carcinogens. Cancer Res. 1988 Aug 15;48(16):4578–4583. [PubMed] [Google Scholar]
- Mita S., Rizzuto R., Moraes C. T., Shanske S., Arnaudo E., Fabrizi G. M., Koga Y., DiMauro S., Schon E. A. Recombination via flanking direct repeats is a major cause of large-scale deletions of human mitochondrial DNA. Nucleic Acids Res. 1990 Feb 11;18(3):561–567. doi: 10.1093/nar/18.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnat R. J., Jr, Loeb L. A. Nucleotide sequence preservation of human mitochondrial DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2895–2899. doi: 10.1073/pnas.82.9.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnat R. J., Jr, Maxwell C. L., Loeb L. A. Nucleotide sequence preservation of human leukemic mitochondrial DNA. Cancer Res. 1985 Apr;45(4):1809–1814. [PubMed] [Google Scholar]
- Monnat R. J., Jr, Reay D. T. Nucleotide sequence identity of mitochondrial DNA from different human tissues. Gene. 1986;43(3):205–211. doi: 10.1016/0378-1119(86)90208-8. [DOI] [PubMed] [Google Scholar]
- Moraes C. T., Ciacci F., Bonilla E., Jansen C., Hirano M., Rao N., Lovelace R. E., Rowland L. P., Schon E. A., DiMauro S. Two novel pathogenic mitochondrial DNA mutations affecting organelle number and protein synthesis. Is the tRNA(Leu(UUR)) gene an etiologic hot spot? J Clin Invest. 1993 Dec;92(6):2906–2915. doi: 10.1172/JCI116913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T., Hibasami H., Maekawa S., Tagawa T., Nakashima K. Preferential binding of cisplatin to mitochondrial DNA and suppression of ATP generation in human malignant melanoma cells. Biochem Int. 1990;20(5):949–955. [PubMed] [Google Scholar]
- Münscher C., Müller-Höcker J., Kadenbach B. Human aging is associated with various point mutations in tRNA genes of mitochondrial DNA. Biol Chem Hoppe Seyler. 1993 Dec;374(12):1099–1104. doi: 10.1515/bchm3.1993.374.7-12.1099. [DOI] [PubMed] [Google Scholar]
- Münscher C., Rieger T., Müller-Höcker J., Kadenbach B. The point mutation of mitochondrial DNA characteristic for MERRF disease is found also in healthy people of different ages. FEBS Lett. 1993 Feb 8;317(1-2):27–30. doi: 10.1016/0014-5793(93)81484-h. [DOI] [PubMed] [Google Scholar]
- Pettepher C. C., LeDoux S. P., Bohr V. A., Wilson G. L. Repair of alkali-labile sites within the mitochondrial DNA of RINr 38 cells after exposure to the nitrosourea streptozotocin. J Biol Chem. 1991 Feb 15;266(5):3113–3117. [PubMed] [Google Scholar]
- Pirsel M., Bohr V. A. Methyl methanesulfonate adduct formation and repair in the DHFR gene and in mitochondrial DNA in hamster cells. Carcinogenesis. 1993 Oct;14(10):2105–2108. doi: 10.1093/carcin/14.10.2105. [DOI] [PubMed] [Google Scholar]
- Poulton J., Bindoff L. A. mtDNA: Pathogenic or nonpathogenic sequence changes. Am J Hum Genet. 1994 Feb;54(2):385–386. [PMC free article] [PubMed] [Google Scholar]
- Pourzand C., Cerutti P. Genotypic mutation analysis by RFLP/PCR. Mutat Res. 1993 Jul;288(1):113–121. doi: 10.1016/0027-5107(93)90213-y. [DOI] [PubMed] [Google Scholar]
- Richter C., Park J. W., Ames B. N. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon E. A., Hirano M., DiMauro S. Mitochondrial encephalomyopathies: clinical and molecular analysis. J Bioenerg Biomembr. 1994 Jun;26(3):291–299. doi: 10.1007/BF00763100. [DOI] [PubMed] [Google Scholar]
- Schon E. A., Rizzuto R., Moraes C. T., Nakase H., Zeviani M., DiMauro S. A direct repeat is a hotspot for large-scale deletion of human mitochondrial DNA. Science. 1989 Apr 21;244(4902):346–349. doi: 10.1126/science.2711184. [DOI] [PubMed] [Google Scholar]
- Seibel P., Degoul F., Romero N., Marsac C., Kadenbach B. Identification of point mutations by mispairing PCR as exemplified in MERRF disease. Biochem Biophys Res Commun. 1990 Dec 14;173(2):561–565. doi: 10.1016/s0006-291x(05)80071-3. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Lezza A. M., Seibel P., Ballinger S. W., Wallace D. C. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell. 1990 Jun 15;61(6):931–937. doi: 10.1016/0092-8674(90)90059-n. [DOI] [PubMed] [Google Scholar]
- Simonetti S., Chen X., DiMauro S., Schon E. A. Accumulation of deletions in human mitochondrial DNA during normal aging: analysis by quantitative PCR. Biochim Biophys Acta. 1992 Dec 10;1180(2):113–122. doi: 10.1016/0925-4439(92)90059-v. [DOI] [PubMed] [Google Scholar]
- Soong N. W., Hinton D. R., Cortopassi G., Arnheim N. Mosaicism for a specific somatic mitochondrial DNA mutation in adult human brain. Nat Genet. 1992 Dec;2(4):318–323. doi: 10.1038/ng1292-318. [DOI] [PubMed] [Google Scholar]
- Sugiyama S., Hattori K., Hayakawa M., Ozawa T. Quantitative analysis of age-associated accumulation of mitochondrial DNA with deletion in human hearts. Biochem Biophys Res Commun. 1991 Oct 31;180(2):894–899. doi: 10.1016/s0006-291x(05)81149-0. [DOI] [PubMed] [Google Scholar]
- Tomkinson A. E., Bonk R. T., Linn S. Mitochondrial endonuclease activities specific for apurinic/apyrimidinic sites in DNA from mouse cells. J Biol Chem. 1988 Sep 5;263(25):12532–12537. [PubMed] [Google Scholar]
- Zeviani M., Moraes C. T., DiMauro S., Nakase H., Bonilla E., Schon E. A., Rowland L. P. Deletions of mitochondrial DNA in Kearns-Sayre syndrome. Neurology. 1988 Sep;38(9):1339–1346. doi: 10.1212/wnl.38.9.1339. [DOI] [PubMed] [Google Scholar]
- Zhang C., Baumer A., Maxwell R. J., Linnane A. W., Nagley P. Multiple mitochondrial DNA deletions in an elderly human individual. FEBS Lett. 1992 Feb 3;297(1-2):34–38. doi: 10.1016/0014-5793(92)80321-7. [DOI] [PubMed] [Google Scholar]
- Zhang C., Linnane A. W., Nagley P. Occurrence of a particular base substitution (3243 A to G) in mitochondrial DNA of tissues of ageing humans. Biochem Biophys Res Commun. 1993 Sep 15;195(2):1104–1110. doi: 10.1006/bbrc.1993.2158. [DOI] [PubMed] [Google Scholar]
- de Vries D. D., van Engelen B. G., Gabreëls F. J., Ruitenbeek W., van Oost B. A. A second missense mutation in the mitochondrial ATPase 6 gene in Leigh's syndrome. Ann Neurol. 1993 Sep;34(3):410–412. doi: 10.1002/ana.410340319. [DOI] [PubMed] [Google Scholar]






