Abstract
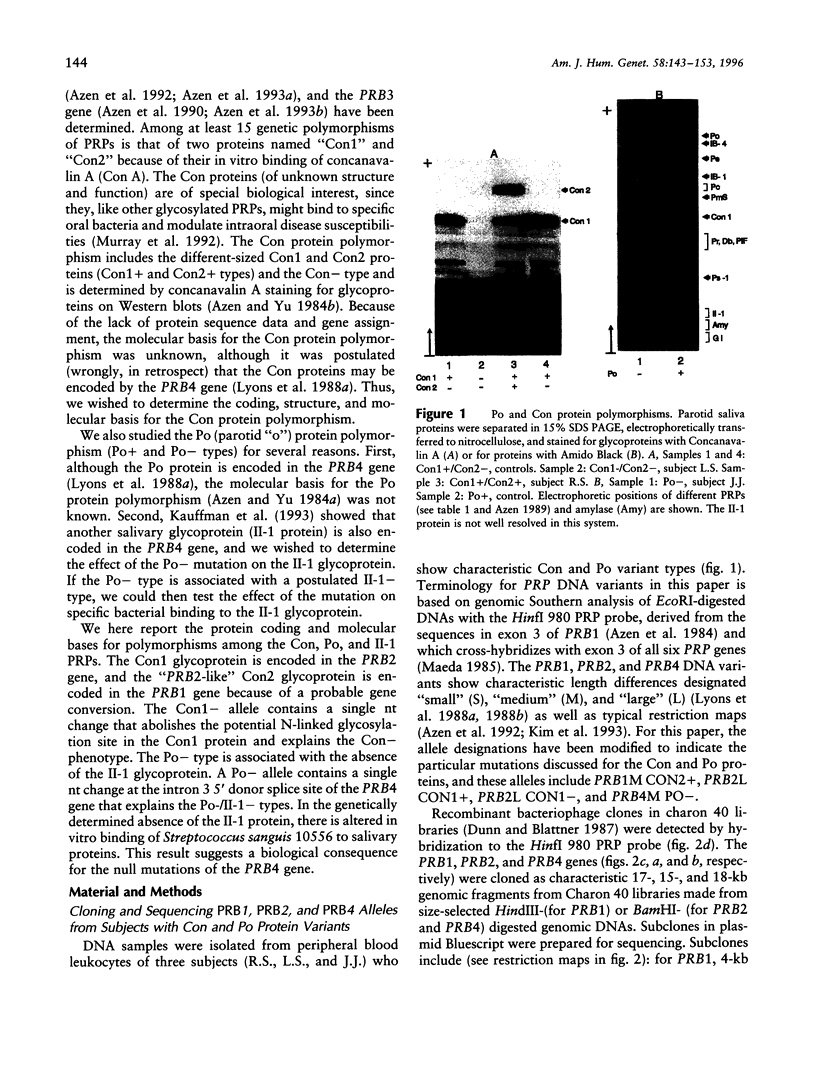
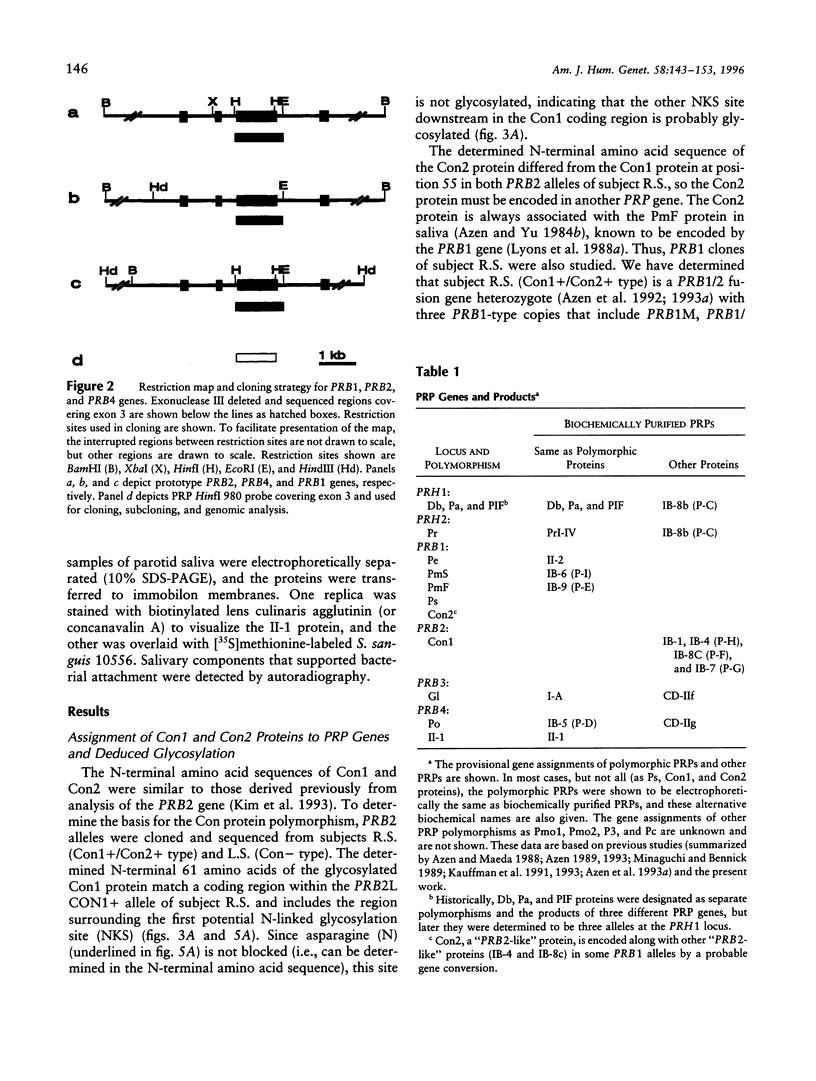
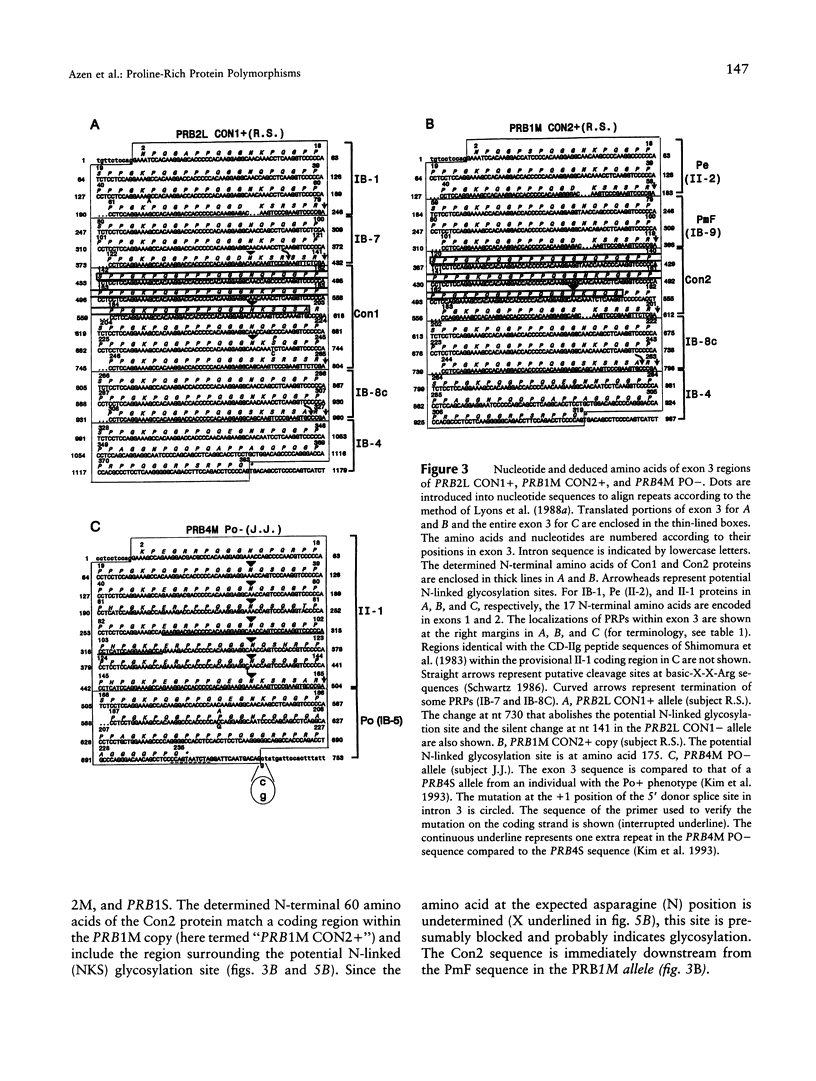
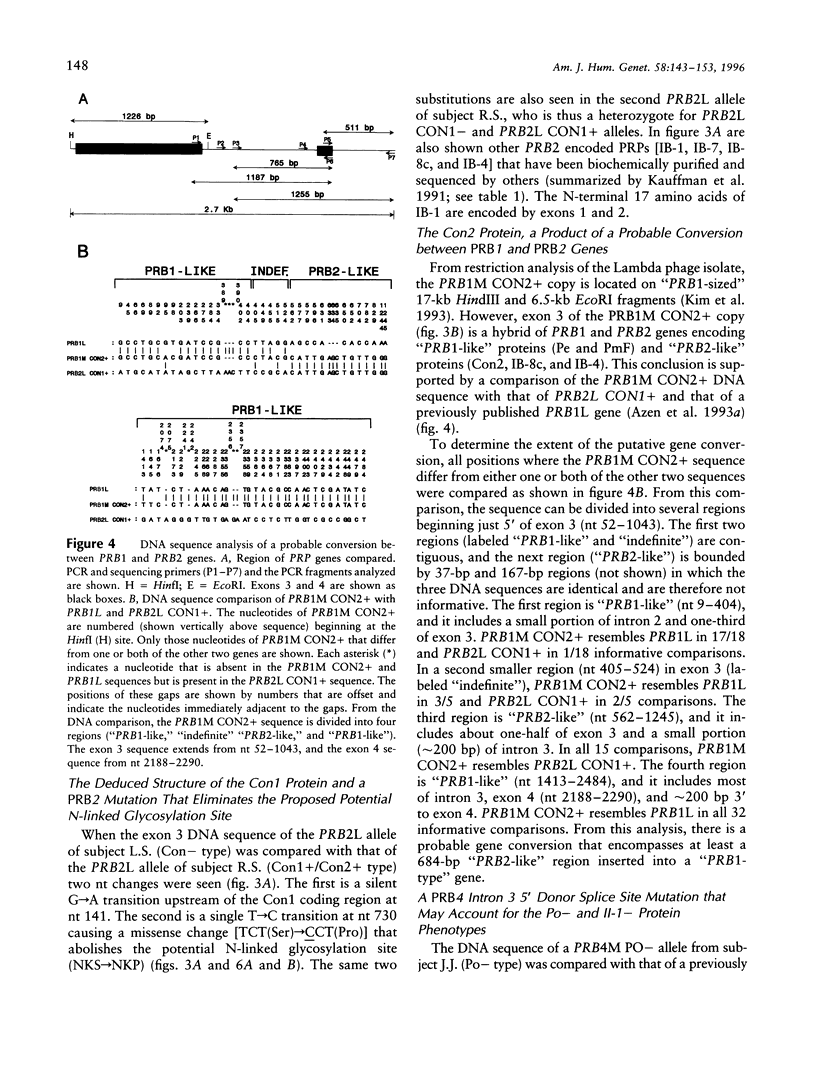
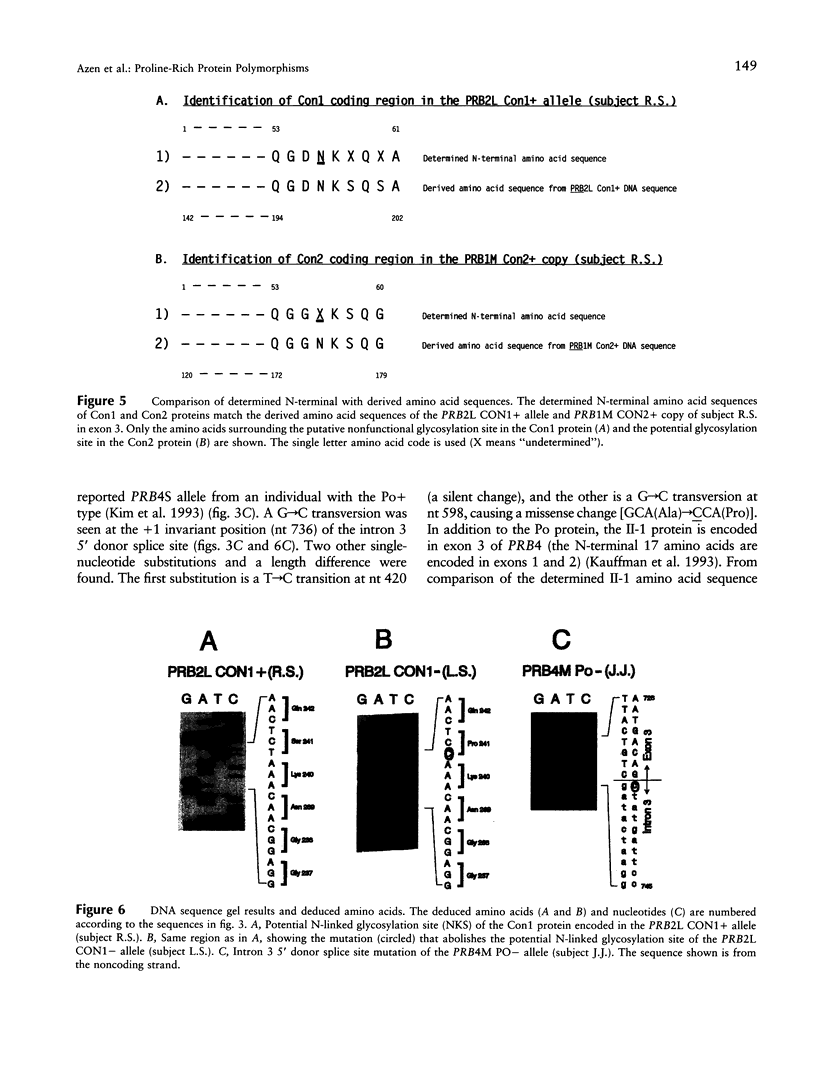
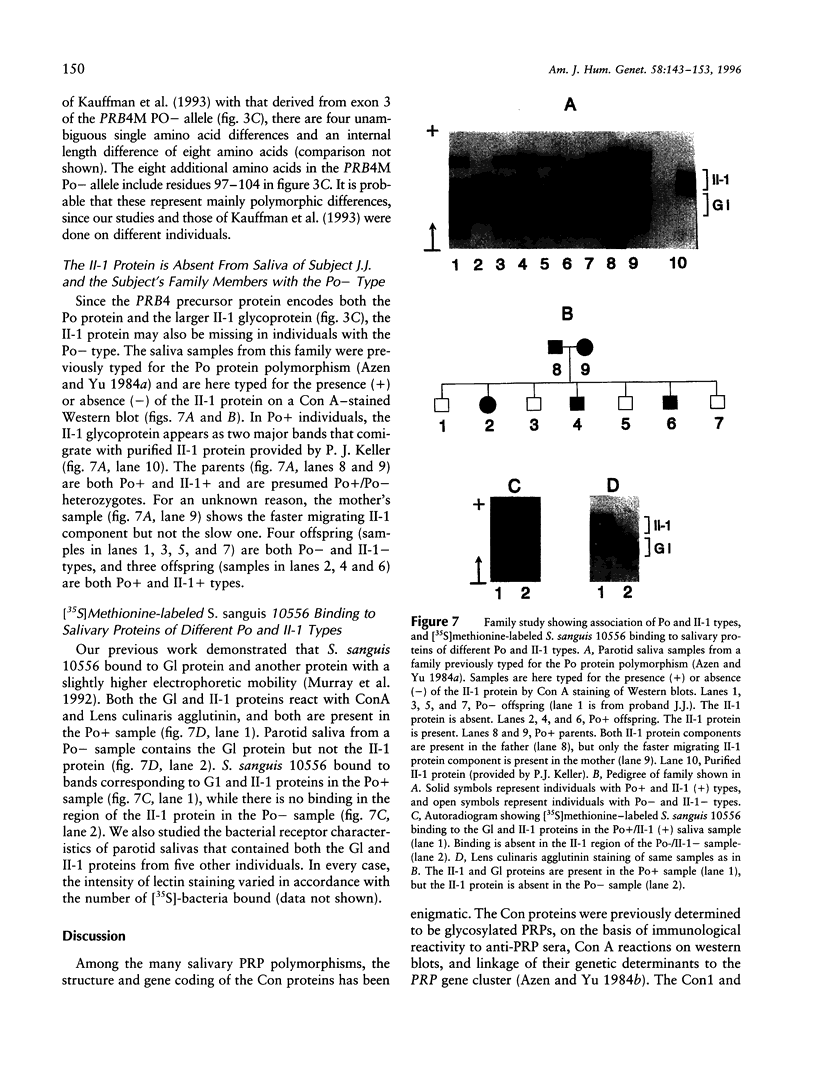
Six closely linked PRP (proline-rich protein) genes code for many salivary PRPs that show frequent length and null variants. From determined protein sequences and DNA sequence analysis of variant alleles, we here report the coding and molecular basis for Con (concanavalin A-binding) and Po (parotid "o") protein polymorphisms. The Con1 glycoprotein is encoded in exon 3 of a PRB2 allele (PRB2L CON1+) with a potential N-linked glycosylation site. Because of a probable gene conversion encompassing > or = 684 bp of DNA, the "PRB2-like" Con2 glycoprotein is encoded in exon 3 of a PRB1 allele (PRB1M CON2+) with a potential glycosylation site. The PmF protein is also encoded in the PRB1M CON2+ allele, thus explaining the previously reported association between Con2 and PmF proteins. A PRB2L CON1 allele contains a single nt missense change [TCT(Ser)-->CCT (Pro)] that abolishes the potential N-linked glycosylation site (NKS-->NKP) in the Con1 protein, and this explains the Con- type. The Po protein and a glycoprotein (II-1) are encoded in the PRB4 gene, and both proteins are absent in the presence of a mutation in the PRB4M PO- allele that contains a single nt change (G--C) at the +1 invariant position of the intron 3 5'donor splice site. The genetically determined absence of the II-1 glycoprotein leads to altered in vitro binding of Streptococcus sanguis 10556 to salivary proteins, which suggests a biological consequence for null mutations of the PRB4 gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azen E. A., Kim H. S., Goodman P., Flynn S., Maeda N. Alleles at the PRH1 locus coding for the human salivary-acidic proline-rich proteins Pa, Db, and PIF. Am J Hum Genet. 1987 Dec;41(6):1035–1047. [PMC free article] [PubMed] [Google Scholar]
- Azen E. A., Latreille P., Niece R. L. PRBI gene variants coding for length and null polymorphisms among human salivary Ps, PmF, PmS, and Pe proline-rich proteins (PRPs). Am J Hum Genet. 1993 Jul;53(1):264–278. [PMC free article] [PubMed] [Google Scholar]
- Azen E. A., Maeda N. Molecular genetics of human salivary proteins and their polymorphisms. Adv Hum Genet. 1988;17:141–199. doi: 10.1007/978-1-4613-0987-1_5. [DOI] [PubMed] [Google Scholar]
- Azen E. A., Minaguchi K., Latreille P., Kim H. S. Alleles at the PRB3 locus coding for a disulfide-bonded human salivary proline-rich glycoprotein (Gl 8) and a null in an Ashkenazi Jew. Am J Hum Genet. 1990 Oct;47(4):686–697. [PMC free article] [PubMed] [Google Scholar]
- Azen E. A., O'Connell P., Kim H. S. PRB2/1 fusion gene: a product of unequal and homologous crossing-over between proline-rich protein (PRP) genes PRB1 and PRB2. Am J Hum Genet. 1992 Apr;50(4):842–851. [PMC free article] [PubMed] [Google Scholar]
- Azen E. A., Yu P. L. Genetic polymorphisms of Pe and Po salivary proteins with probable linkage of their genes to the salivary protein gene complex (SPC). Biochem Genet. 1984 Dec;22(11-12):1065–1080. doi: 10.1007/BF00499632. [DOI] [PubMed] [Google Scholar]
- Azen E., Lyons K. M., McGonigal T., Barrett N. L., Clements L. S., Maeda N., Vanin E. F., Carlson D. M., Smithies O. Clones from the human gene complex coding for salivary proline-rich proteins. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5561–5565. doi: 10.1073/pnas.81.17.5561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azen E., Prakobphol A., Fisher S. J. PRB3 null mutations result in absence of the proline-rich glycoprotein Gl and abolish Fusobacterium nucleatum interactions with saliva in vitro. Infect Immun. 1993 Oct;61(10):4434–4439. doi: 10.1128/iai.61.10.4434-4439.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag R. J., Waldman A. S., Liskay R. M. Homologous recombination in mammalian cells. Annu Rev Genet. 1989;23:199–225. doi: 10.1146/annurev.ge.23.120189.001215. [DOI] [PubMed] [Google Scholar]
- Dunn I. S., Blattner F. R. Charons 36 to 40: multi enzyme, high capacity, recombination deficient replacement vectors with polylinkers and polystuffers. Nucleic Acids Res. 1987 Mar 25;15(6):2677–2698. doi: 10.1093/nar/15.6.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavel Y., von Heijne G. Sequence differences between glycosylated and non-glycosylated Asn-X-Thr/Ser acceptor sites: implications for protein engineering. Protein Eng. 1990 Apr;3(5):433–442. doi: 10.1093/protein/3.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillece-Castro B. L., Prakobphol A., Burlingame A. L., Leffler H., Fisher S. J. Structure and bacterial receptor activity of a human salivary proline-rich glycoprotein. J Biol Chem. 1991 Sep 15;266(26):17358–17368. [PubMed] [Google Scholar]
- Hay D. I., Ahern J. M., Schluckebier S. K., Schlesinger D. H. Human salivary acidic proline-rich protein polymorphisms and biosynthesis studied by high-performance liquid chromatography. J Dent Res. 1994 Nov;73(11):1717–1726. doi: 10.1177/00220345940730110701. [DOI] [PubMed] [Google Scholar]
- Kauffman D. L., Bennick A., Blum M., Keller P. J. Basic proline-rich proteins from human parotid saliva: relationships of the covalent structures of ten proteins from a single individual. Biochemistry. 1991 Apr 9;30(14):3351–3356. doi: 10.1021/bi00228a001. [DOI] [PubMed] [Google Scholar]
- Kauffman D. L., Keller P. J., Bennick A., Blum M. Alignment of amino acid and DNA sequences of human proline-rich proteins. Crit Rev Oral Biol Med. 1993;4(3-4):287–292. doi: 10.1177/10454411930040030501. [DOI] [PubMed] [Google Scholar]
- Kim H. S., Lyons K. M., Saitoh E., Azen E. A., Smithies O., Maeda N. The structure and evolution of the human salivary proline-rich protein gene family. Mamm Genome. 1993;4(1):3–14. doi: 10.1007/BF00364656. [DOI] [PubMed] [Google Scholar]
- Kim H. S., Smithies O., Maeda N. A physical map of the human salivary proline-rich protein gene cluster covers over 700 kbp of DNA. Genomics. 1990 Feb;6(2):260–267. doi: 10.1016/0888-7543(90)90565-c. [DOI] [PubMed] [Google Scholar]
- Krawczak M., Reiss J., Cooper D. N. The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: causes and consequences. Hum Genet. 1992 Sep-Oct;90(1-2):41–54. doi: 10.1007/BF00210743. [DOI] [PubMed] [Google Scholar]
- Lyons K. M., Azen E. A., Goodman P. A., Smithies O. Many protein products from a few loci: assignment of human salivary proline-rich proteins to specific loci. Genetics. 1988 Sep;120(1):255–265. doi: 10.1093/genetics/120.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons K. M., Stein J. H., Smithies O. Length polymorphisms in human proline-rich protein genes generated by intragenic unequal crossing over. Genetics. 1988 Sep;120(1):267–278. doi: 10.1093/genetics/120.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N. Inheritance of the human salivary proline-rich proteins: a reinterpretation in terms of six loci forming two subfamilies. Biochem Genet. 1985 Jun;23(5-6):455–464. doi: 10.1007/BF00499086. [DOI] [PubMed] [Google Scholar]
- Maeda N., Kim H. S., Azen E. A., Smithies O. Differential RNA splicing and post-translational cleavages in the human salivary proline-rich protein gene system. J Biol Chem. 1985 Sep 15;260(20):11123–11130. [PubMed] [Google Scholar]
- Minaguchi K., Bennick A. Genetics of human salivary proteins. J Dent Res. 1989 Jan;68(1):2–15. doi: 10.1177/00220345890680010201. [DOI] [PubMed] [Google Scholar]
- Murray P. A., Prakobphol A., Lee T., Hoover C. I., Fisher S. J. Adherence of oral streptococci to salivary glycoproteins. Infect Immun. 1992 Jan;60(1):31–38. doi: 10.1128/iai.60.1.31-38.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz T. W. The processing of peptide precursors. 'Proline-directed arginyl cleavage' and other monobasic processing mechanisms. FEBS Lett. 1986 May 5;200(1):1–10. doi: 10.1016/0014-5793(86)80500-2. [DOI] [PubMed] [Google Scholar]
- Shimomura H., Kanai Y., Sanada K. Amino acid sequences of glycopeptides obtained from basic proline-rich glycoprotein of human parotid saliva. J Biochem. 1983 Mar;93(3):857–863. doi: 10.1093/jb/93.3.857. [DOI] [PubMed] [Google Scholar]