Abstract
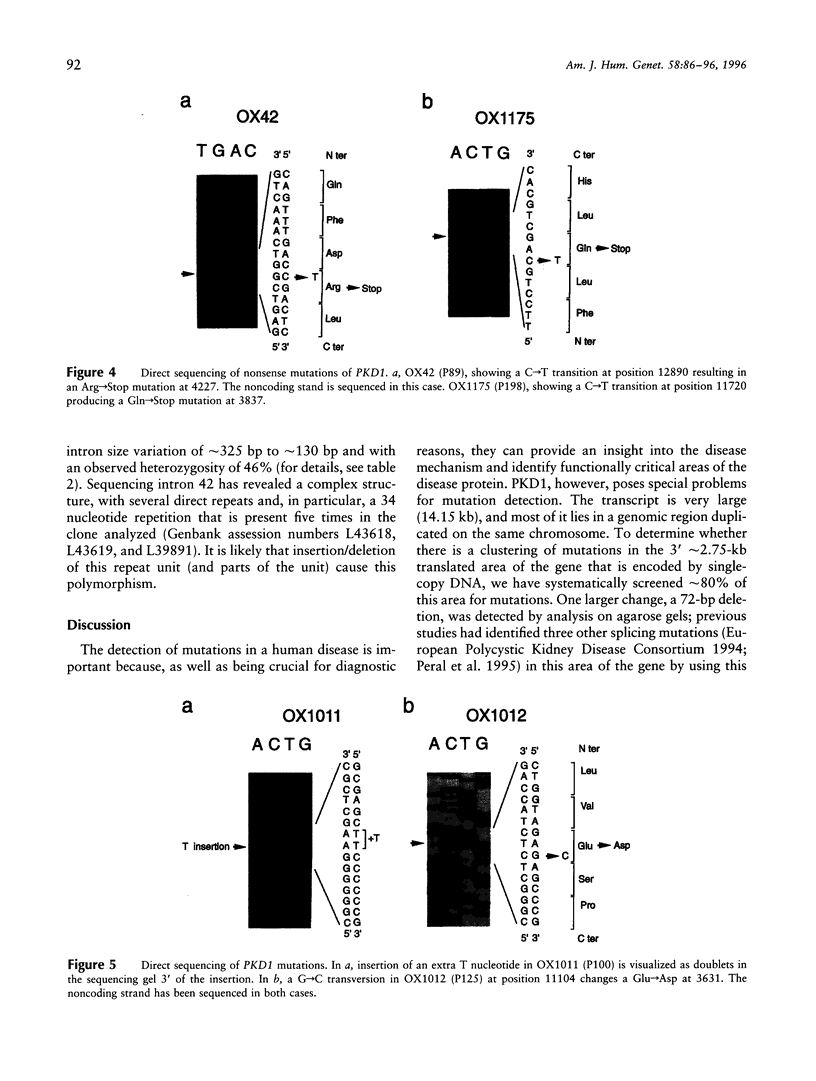
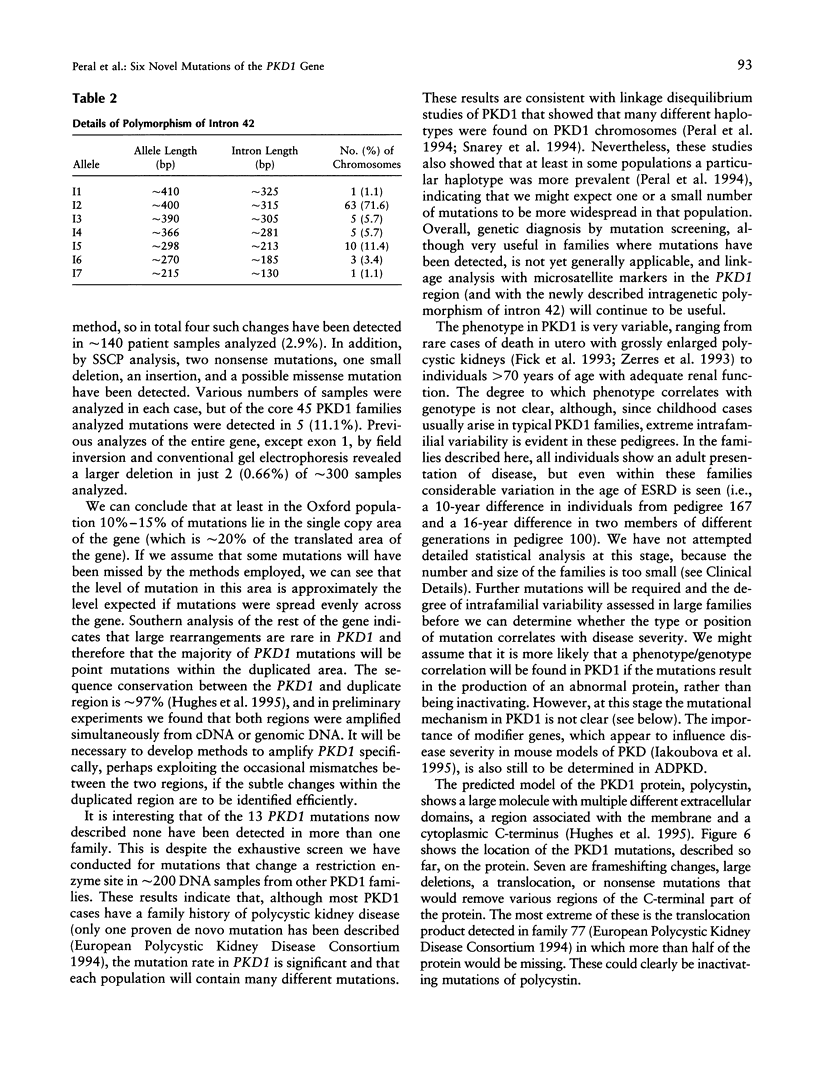
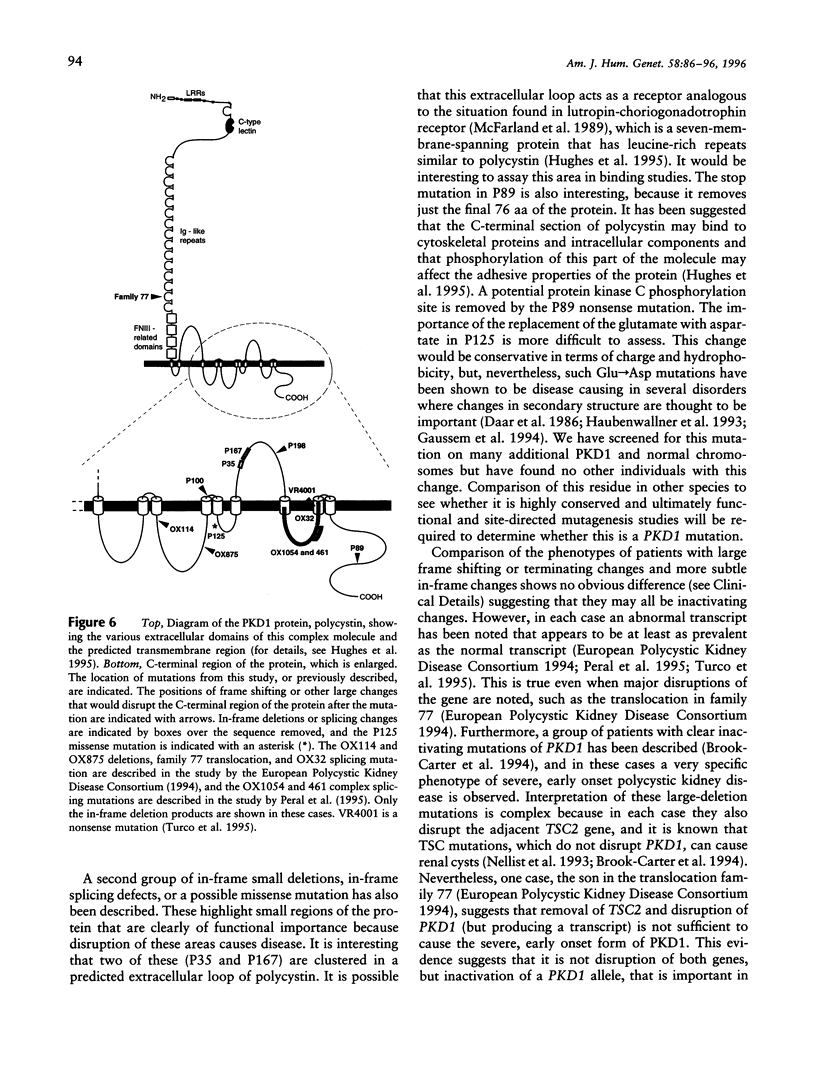
Recently, the gene for the most common form of autosomal dominant polycystic kidney disease (ADPKD), PKD1 (polycystic kidney disease 1), has been fully characterized and shown to encode an integral membrane protein, polycystin, involved in cell-cell and/or cell-matrix interactions. Study of the PKD1 gene has been complicated because most of the gene lies in a genomic region reiterated several times elsewhere on the same chromosome, and consequently only seven mutations have been described so far. Here we report a systematic screen covering approximately 80% of the approximately 2.75 kb of translated transcript that is encoded by single-copy DNA. We have identified and characterized six novel mutations that, together with the previously described changes, amount to a detection rate of 10%-15% in the population studied. The newly described mutations are two deletions, an insertion of a T-nucleotide causing a frame shift, two single-base-pair substitutions resulting in premature stop codons, and a G-->C transversion that may be a missense mutation. These results have important implications for genetic diagnosis of PKD1 because they indicate that the majority of mutations lie within the duplicated area, which is difficult to study. The regions of polycystin removed in each mutation so far described are assessed for their functional significance; an area disrupted by two new small in-frame changes is highlighted. PKD1 mutations are contrasted with those in the PKD1/TSC2 contiguous-gene syndrome, and the likely mutational mechanism in PKD1 is considered.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bogdanova N., Dworniczak B., Dragova D., Todorov V., Dimitrakov D., Kalinov K., Hallmayer J., Horst J., Kalaydjieva L. Genetic heterogeneity of polycystic kidney disease in Bulgaria. Hum Genet. 1995 Jun;95(6):645–650. doi: 10.1007/BF00209481. [DOI] [PubMed] [Google Scholar]
- Brook-Carter P. T., Peral B., Ward C. J., Thompson P., Hughes J., Maheshwar M. M., Nellist M., Gamble V., Harris P. C., Sampson J. R. Deletion of the TSC2 and PKD1 genes associated with severe infantile polycystic kidney disease--a contiguous gene syndrome. Nat Genet. 1994 Dec;8(4):328–332. doi: 10.1038/ng1294-328. [DOI] [PubMed] [Google Scholar]
- Burn T. C., Connors T. D., Dackowski W. R., Petry L. R., Van Raay T. J., Millholland J. M., Venet M., Miller G., Hakim R. M., Landes G. M. Analysis of the genomic sequence for the autosomal dominant polycystic kidney disease (PKD1) gene predicts the presence of a leucine-rich repeat. The American PKD1 Consortium (APKD1 Consortium). Hum Mol Genet. 1995 Apr;4(4):575–582. doi: 10.1093/hmg/4.4.575. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Daar I. O., Artymiuk P. J., Phillips D. C., Maquat L. E. Human triose-phosphate isomerase deficiency: a single amino acid substitution results in a thermolabile enzyme. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7903–7907. doi: 10.1073/pnas.83.20.7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoust M. C., Reynolds D. M., Bichet D. G., Somlo S. Evidence for a third genetic locus for autosomal dominant polycystic kidney disease. Genomics. 1995 Feb 10;25(3):733–736. doi: 10.1016/0888-7543(95)80020-m. [DOI] [PubMed] [Google Scholar]
- Dodé C., Rochette J., Krishnamoorthy R. Locus assignment of human alpha globin mutations by selective amplification and direct sequencing. Br J Haematol. 1990 Oct;76(2):275–281. doi: 10.1111/j.1365-2141.1990.tb07884.x. [DOI] [PubMed] [Google Scholar]
- Fick G. M., Johnson A. M., Gabow P. A. Is there evidence for anticipation in autosomal-dominant polycystic kidney disease? Kidney Int. 1994 Apr;45(4):1153–1162. doi: 10.1038/ki.1994.153. [DOI] [PubMed] [Google Scholar]
- Fick G. M., Johnson A. M., Strain J. D., Kimberling W. J., Kumar S., Manco-Johnson M. L., Duley I. T., Gabow P. A. Characteristics of very early onset autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1993 Jun;3(12):1863–1870. doi: 10.1681/ASN.V3121863. [DOI] [PubMed] [Google Scholar]
- Gabow P. A. Autosomal dominant polycystic kidney disease--more than a renal disease. Am J Kidney Dis. 1990 Nov;16(5):403–413. doi: 10.1016/s0272-6386(12)80051-5. [DOI] [PubMed] [Google Scholar]
- Gaussem P., Gandrille S., Duchemin J., Emmerich J., Alhenc-Gelas M., Aillaud M. F., Aiach M. Influence of six mutations of the protein C gene on the Gla domain conformation and calcium affinity. Thromb Haemost. 1994 Jun;71(6):748–754. [PubMed] [Google Scholar]
- Glavac D., Dean M. Optimization of the single-strand conformation polymorphism (SSCP) technique for detection of point mutations. Hum Mutat. 1993;2(5):404–414. doi: 10.1002/humu.1380020513. [DOI] [PubMed] [Google Scholar]
- Harris P. C., Thomas S., Ratcliffe P. J., Breuning M. H., Coto E., Lopez-Larrea C. Rapid genetic analysis of families with polycystic kidney disease 1 by means of a microsatellite marker. Lancet. 1991 Dec 14;338(8781):1484–1487. doi: 10.1016/0140-6736(91)92300-q. [DOI] [PubMed] [Google Scholar]
- Haubenwallner S., Hörl G., Shachter N. S., Presta E., Fried S. K., Höfler G., Kostner G. M., Breslow J. L., Zechner R. A novel missense mutation in the gene for lipoprotein lipase resulting in a highly conservative amino acid substitution (Asp180-->Glu) causes familial chylomicronemia (type I hyperlipoproteinemia). Genomics. 1993 Nov;18(2):392–396. doi: 10.1006/geno.1993.1481. [DOI] [PubMed] [Google Scholar]
- Hughes J., Ward C. J., Peral B., Aspinwall R., Clark K., San Millán J. L., Gamble V., Harris P. C. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet. 1995 Jun;10(2):151–160. doi: 10.1038/ng0695-151. [DOI] [PubMed] [Google Scholar]
- Iakoubova O. A., Dushkin H., Beier D. R. Localization of a murine recessive polycystic kidney disease mutation and modifying loci that affect disease severity. Genomics. 1995 Mar 1;26(1):107–114. doi: 10.1016/0888-7543(95)80088-4. [DOI] [PubMed] [Google Scholar]
- Kimberling W. J., Kumar S., Gabow P. A., Kenyon J. B., Connolly C. J., Somlo S. Autosomal dominant polycystic kidney disease: localization of the second gene to chromosome 4q13-q23. Genomics. 1993 Dec;18(3):467–472. doi: 10.1016/s0888-7543(11)80001-7. [DOI] [PubMed] [Google Scholar]
- Milutinovic J., Rust P. F., Fialkow P. J., Agodoa L. Y., Phillips L. A., Rudd T. G., Sutherland S. Intrafamilial phenotypic expression of autosomal dominant polycystic kidney disease. Am J Kidney Dis. 1992 May;19(5):465–472. doi: 10.1016/s0272-6386(12)80956-5. [DOI] [PubMed] [Google Scholar]
- Nellist M., Brook-Carter P. T., Connor J. M., Kwiatkowski D. J., Johnson P., Sampson J. R. Identification of markers flanking the tuberous sclerosis locus on chromosome 9 (TSC1). J Med Genet. 1993 Mar;30(3):224–227. doi: 10.1136/jmg.30.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peral B., Gamble V., San Millán J. L., Strong C., Sloane-Stanley J., Moreno F., Harris P. C. Splicing mutations of the polycystic kidney disease 1 (PKD1) gene induced by intronic deletion. Hum Mol Genet. 1995 Apr;4(4):569–574. doi: 10.1093/hmg/4.4.569. [DOI] [PubMed] [Google Scholar]
- Peral B., Ward C. J., San Millán J. L., Thomas S., Stallings R. L., Moreno F., Harris P. C. Evidence of linkage disequilibrium in the Spanish polycystic kidney disease I population. Am J Hum Genet. 1994 May;54(5):899–908. [PMC free article] [PubMed] [Google Scholar]
- Peters D. J., Sandkuijl L. A. Genetic heterogeneity of polycystic kidney disease in Europe. Contrib Nephrol. 1992;97:128–139. doi: 10.1159/000421651. [DOI] [PubMed] [Google Scholar]
- Peters D. J., Spruit L., Saris J. J., Ravine D., Sandkuijl L. A., Fossdal R., Boersma J., van Eijk R., Nørby S., Constantinou-Deltas C. D. Chromosome 4 localization of a second gene for autosomal dominant polycystic kidney disease. Nat Genet. 1993 Dec;5(4):359–362. doi: 10.1038/ng1293-359. [DOI] [PubMed] [Google Scholar]
- Ravine D., Walker R. G., Gibson R. N., Forrest S. M., Richards R. I., Friend K., Sheffield L. J., Kincaid-Smith P., Danks D. M. Phenotype and genotype heterogeneity in autosomal dominant polycystic kidney disease. Lancet. 1992 Nov 28;340(8831):1330–1333. doi: 10.1016/0140-6736(92)92503-8. [DOI] [PubMed] [Google Scholar]
- Reeders S. T., Breuning M. H., Davies K. E., Nicholls R. D., Jarman A. P., Higgs D. R., Pearson P. L., Weatherall D. J. A highly polymorphic DNA marker linked to adult polycystic kidney disease on chromosome 16. Nature. 1985 Oct 10;317(6037):542–544. doi: 10.1038/317542a0. [DOI] [PubMed] [Google Scholar]
- Ryynanen M., Dolata M. M., Lampainen E., Reeders S. T. Localisation of a mutation producing autosomal dominant polycystic kidney disease without renal failure. J Med Genet. 1987 Aug;24(8):462–465. doi: 10.1136/jmg.24.8.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snarey A., Thomas S., Schneider M. C., Pound S. E., Barton N., Wright A. F., Somlo S., Germino G. G., Harris P. C., Reeders S. T. Linkage disequilibrium in the region of the autosomal dominant polycystic kidney disease gene (PKD1). Am J Hum Genet. 1994 Aug;55(2):365–371. [PMC free article] [PubMed] [Google Scholar]
- Spinardi L., Mazars R., Theillet C. Protocols for an improved detection of point mutations by SSCP. Nucleic Acids Res. 1991 Jul 25;19(14):4009–4009. doi: 10.1093/nar/19.14.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thein S. L., Hinton J. A simple and rapid method of direct sequencing using Dynabeads. Br J Haematol. 1991 Sep;79(1):113–115. doi: 10.1111/j.1365-2141.1991.tb08016.x. [DOI] [PubMed] [Google Scholar]
- Turco A. E., Rossetti S., Bresin E., Corra S., Gammaro L., Maschio G., Pignatti P. F. A novel nonsense mutation in the PKD1 gene (C3817T) is associated with autosomal dominant polycystic kidney disease (ADPKD) in a large three-generation Italian family. Hum Mol Genet. 1995 Aug;4(8):1331–1335. doi: 10.1093/hmg/4.8.1331. [DOI] [PubMed] [Google Scholar]
- Zerres K., Rudnik-Schöneborn S., Deget F. Childhood onset autosomal dominant polycystic kidney disease in sibs: clinical picture and recurrence risk. German Working Group on Paediatric Nephrology (Arbeitsgemeinschaft für Pädiatrische Nephrologie. J Med Genet. 1993 Jul;30(7):583–588. doi: 10.1136/jmg.30.7.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida S., de Almeida E., Peters D., Pinto J. R., Távora I., Lavinha J., Breuning M., Prata M. M. Autosomal dominant polycystic kidney disease: evidence for the existence of a third locus in a Portuguese family. Hum Genet. 1995 Jul;96(1):83–88. doi: 10.1007/BF00214191. [DOI] [PubMed] [Google Scholar]