Abstract
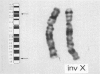
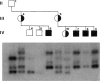
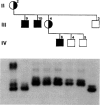
It has been demonstrated in animal studies that, in animals heterozygous for pericentric chromosomal inversions, loop formation is greatly reduced during meiosis. This results in absence of recombination within the inverted segment, with recombination seen only outside the inversion. A recent study in yeast has shown that telomeres, rather than centromeres, lead in chromosome movement just prior to meiosis and may be involved in promoting recombination. We studied by cytogenetic analysis and DNA polymorphisms the nature of meiotic recombination in a three-generation family with a large pericentric X chromosome inversion, inv(X)(p21.1q26), in which Duchenne muscular dystrophy (DMD) was cosegregating with the inversion. On DNA analysis there was no evidence of meiotic recombination between the inverted and normal X chromosomes in the inverted segment. Recombination was seen at the telomeric regions, Xp22 and Xq27-28. No deletion or point mutation was found on analysis of the DMD gene. On the basis of the FISH results, we believe that the X inversion is the mutation responsible for DMD in this family. Our results indicate that (1) pericentric X chromosome inversions result in reduction of recombination between the normal and inverted X chromosomes; (2) meiotic X chromosome pairing in these individuals is likely initiated at the telomeres; and (3) in this family DMD is caused by the pericentric inversion.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allderdice P. W., Browne N., Murphy D. P. Chromosome 3 duplication q21 leads to qter deletion p25 leads to pter syndrome in children of carriers of a pericentric inversion inv(3) (p25q21). Am J Hum Genet. 1975 Nov;27(6):699–718. [PMC free article] [PubMed] [Google Scholar]
- Ashley T., Cacheiro N. L. Correlation between meiotic behavior and breakpoints with respect to G-bands in two X-4 mouse translocations: T(X;4)7R1 and T(X;4)8R1. Cytogenet Cell Genet. 1990;53(4):178–184. doi: 10.1159/000132926. [DOI] [PubMed] [Google Scholar]
- Ashley T., Cacheiro N. L., Russell L. B., Ward D. C. Molecular characterization of a pericentric inversion in mouse chromosome 8 implicates telomeres as promoters of meiotic recombination. Chromosoma. 1993 Jan;102(2):112–120. doi: 10.1007/BF00356028. [DOI] [PubMed] [Google Scholar]
- Ashley T., Moses M. J., Solari A. J. Fine structure and behaviour of a pericentric inversion in the sand rat, Psammomys obesus. J Cell Sci. 1981 Aug;50:105–119. doi: 10.1242/jcs.50.1.105. [DOI] [PubMed] [Google Scholar]
- Ashley T. Prediction of mammalian meiotic synaptic and recombinational behavior of inversion heterozygotes based on mitotic breakpoint data and the possible evolutionary consequences. Genetica. 1990;83(1):1–7. doi: 10.1007/BF00774683. [DOI] [PubMed] [Google Scholar]
- Beggs A. H., Koenig M., Boyce F. M., Kunkel L. M. Detection of 98% of DMD/BMD gene deletions by polymerase chain reaction. Hum Genet. 1990 Nov;86(1):45–48. doi: 10.1007/BF00205170. [DOI] [PubMed] [Google Scholar]
- Boyd H., Kaste J., Hovi E., Ritanen-Mohammed U. M., Käriäinen H., de la Chapelle A., Lehesjoki A. E. Familial pericentric inversion inv(8)(p23q11). J Med Genet. 1994 Mar;31(3):201–205. doi: 10.1136/jmg.31.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd Y., Buckle V., Holt S., Munro E., Hunter D., Craig I. Muscular dystrophy in girls with X;autosome translocations. J Med Genet. 1986 Dec;23(6):484–490. doi: 10.1136/jmg.23.6.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd Y., Cockburn D., Holt S., Munro E., Van Ommen G. J., Gillard B., Affara N., Ferguson-Smith M., Craig I. Mapping of 12 translocation breakpoints in the Xp21 region with respect to the locus for Duchenne muscular dystrophy. Cytogenet Cell Genet. 1988;48(1):28–34. doi: 10.1159/000132581. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. S., Gibbs R. A., Ranier J. E., Nguyen P. N., Caskey C. T. Deletion screening of the Duchenne muscular dystrophy locus via multiplex DNA amplification. Nucleic Acids Res. 1988 Dec 9;16(23):11141–11156. doi: 10.1093/nar/16.23.11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandley A. C., McBeath S., Speed R. M., Yorston L., Hargreave T. B. Pericentric inversion in human chromosome 1 and the risk for male sterility. J Med Genet. 1987 Jun;24(6):325–334. doi: 10.1136/jmg.24.6.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikashige Y., Ding D. Q., Funabiki H., Haraguchi T., Mashiko S., Yanagida M., Hiraoka Y. Telomere-led premeiotic chromosome movement in fission yeast. Science. 1994 Apr 8;264(5156):270–273. doi: 10.1126/science.8146661. [DOI] [PubMed] [Google Scholar]
- Clemens P. R., Fenwick R. G., Chamberlain J. S., Gibbs R. A., de Andrade M., Chakraborty R., Caskey C. T. Carrier detection and prenatal diagnosis in Duchenne and Becker muscular dystrophy families, using dinucleotide repeat polymorphisms. Am J Hum Genet. 1991 Nov;49(5):951–960. [PMC free article] [PubMed] [Google Scholar]
- Coyne J. A., Aulard S., Berry A. Lack of underdominance in a naturally occurring pericentric inversion in Drosophila melanogaster and its implications for chromosome evolution. Genetics. 1991 Nov;129(3):791–802. doi: 10.1093/genetics/129.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne J. A., Meyers W., Crittenden A. P., Sniegowski P. The fertility effects of pericentric inversions in Drosophila melanogaster. Genetics. 1993 Jun;134(2):487–496. doi: 10.1093/genetics/134.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel A. Structural differences in pericentric inversions. Application to a model of risk of recombinants. Hum Genet. 1981;56(3):321–328. doi: 10.1007/BF00274687. [DOI] [PubMed] [Google Scholar]
- Disteche C. M., McConnell G. K., Grant S. G., Stephenson D. A., Chapman V. M., Gandy S., Adler D. A. Comparison of the physical and recombination maps of the mouse X chromosome. Genomics. 1989 Aug;5(2):177–184. doi: 10.1016/0888-7543(89)90044-x. [DOI] [PubMed] [Google Scholar]
- Duckett D. P., Young I. D. A recombinant X chromosome in a short statured girl resulting from a maternal pericentric inversion. Hum Genet. 1988 Jul;79(3):251–254. doi: 10.1007/BF00366246. [DOI] [PubMed] [Google Scholar]
- Faed M. J., Lamont M. A., Baxby K. Cytogenetic and histological studies of testicular biopsies from subfertile men with chromosome anomaly. J Med Genet. 1982 Feb;19(1):49–56. doi: 10.1136/jmg.19.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel-Robez O., Ratomponirina C., Croquette M., Couturier J., Rumpler Y. Synaptonemal complexes in a subfertile man with a pericentric inversion in chromosome 21. Heterosynapsis without previous homosynapsis. Cytogenet Cell Genet. 1988;48(2):84–87. doi: 10.1159/000132595. [DOI] [PubMed] [Google Scholar]
- Gabriel-Robez O., Ratomponirina C., Croquette M., Maetz J. L., Couturier J., Rumpler Y. Reproductive failure and pericentric inversion in man. Andrologia. 1987 Nov-Dec;19(6):662–669. doi: 10.1111/j.1439-0272.1987.tb01924.x. [DOI] [PubMed] [Google Scholar]
- Gabriel-Robez O., Ratomponirina C., Rumpler Y., Le Marec B., Luciani J. M., Guichaoua M. R. Synapsis and synaptic adjustment in an infertile human male heterozygous for a pericentric inversion in chromosome 1. Hum Genet. 1986 Feb;72(2):148–152. doi: 10.1007/BF00283934. [DOI] [PubMed] [Google Scholar]
- Golden W. L., von Kap-Herr C., Kurth B., Wright R. M., Flickinger C. J., Eddy R., Shows T., Herr J. C. Refinement of the localization of the gene for human intraacrosomal protein SP-10 (ACRV1) to the junction of bands q23-->q24 of chromosome 11 by nonisotopic in situ hybridization. Genomics. 1993 Nov;18(2):446–449. doi: 10.1006/geno.1993.1496. [DOI] [PubMed] [Google Scholar]
- Greenbaum I. F., Reed M. J. Evidence for heterosynaptic pairing of the inverted segment in pericentric inversion heterozygotes of the deer mouse (Peromyscus maniculatus). Cytogenet Cell Genet. 1984;38(2):106–111. doi: 10.1159/000132040. [DOI] [PubMed] [Google Scholar]
- Hale D. W. Heterosynapsis and suppression of chiasmata within heterozygous pericentric inversions of the Sitka deer mouse. Chromosoma. 1986;94(6):425–432. doi: 10.1007/BF00292751. [DOI] [PubMed] [Google Scholar]
- Hartley D. A., Davies K. E., Drayna D., White R. L., Williamson R. A cytological map of the human X chromosome--evidence for non-random recombination. Nucleic Acids Res. 1984 Jul 11;12(13):5277–5285. doi: 10.1093/nar/12.13.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke A., Fischer C., Rappold G. A. Genetic map of the human pseudoautosomal region reveals a high rate of recombination in female meiosis at the Xp telomere. Genomics. 1993 Dec;18(3):478–485. doi: 10.1016/s0888-7543(11)80003-0. [DOI] [PubMed] [Google Scholar]
- Hirsch B., Baldinger S. Pericentric inversion of chromosome 4 giving rise to dup(4p) and dup(4q) recombinants within a single kindred. Am J Med Genet. 1993 Jan 1;45(1):5–8. doi: 10.1002/ajmg.1320450104. [DOI] [PubMed] [Google Scholar]
- Huang T. H., Cottingham R. W., Jr, Ledbetter D. H., Zoghbi H. Y. Genetic mapping of four dinucleotide repeat loci, DXS453, DXS458, DXS454, and DXS424, on the X chromosome using multiplex polymerase chain reaction. Genomics. 1992 Jun;13(2):375–380. doi: 10.1016/0888-7543(92)90256-r. [DOI] [PubMed] [Google Scholar]
- Jacobs P. A., Hunt P. A., Mayer M., Bart R. D. Duchenne muscular dystrophy (DMD) in a female with an X/autosome translocation: further evidence that the DMD locus is at Xp21. Am J Hum Genet. 1981 Jul;33(4):513–518. [PMC free article] [PubMed] [Google Scholar]
- Kaelbling M., Fechheimer N. S. Synaptonemal complex analysis of a pericentric inversion in chromosome 2 of domestic fowl, Gallus domesticus. Cytogenet Cell Genet. 1985;39(2):82–86. doi: 10.1159/000132112. [DOI] [PubMed] [Google Scholar]
- Kaiser P. Pericentric inversions. Problems and significance for clinical genetics. Hum Genet. 1984;68(1):1–47. doi: 10.1007/BF00293869. [DOI] [PubMed] [Google Scholar]
- Koenig M., Hoffman E. P., Bertelson C. J., Monaco A. P., Feener C., Kunkel L. M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987 Jul 31;50(3):509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Konagaya M., Honda H., Sakai M., Iida M. Transmission of dystrophinopathy by X-chromosome inversion. Neurology. 1995 Jul;45(7):1409–1410. doi: 10.1212/wnl.45.7.1409. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg L., Pelto K., Borgström G. H. Familial pericentric inversion (3)(p12q24). Hum Genet. 1992 Jun;89(4):433–436. doi: 10.1007/BF00194317. [DOI] [PubMed] [Google Scholar]
- Martin R. H. Cytogenetic analysis of sperm from a man heterozygous for a pericentric inversion, inv (3) (p25q21). Am J Hum Genet. 1991 May;48(5):856–861. [PMC free article] [PubMed] [Google Scholar]
- Matise T. C., Perlin M., Chakravarti A. Automated construction of genetic linkage maps using an expert system (MultiMap): a human genome linkage map. Nat Genet. 1994 Apr;6(4):384–390. doi: 10.1038/ng0494-384. [DOI] [PubMed] [Google Scholar]
- Ram K. T., Barker D. F., Puck J. M. Dinucleotide repeat polymorphism at the DXS441 locus. Nucleic Acids Res. 1992 Mar 25;20(6):1428–1428. doi: 10.1093/nar/20.6.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. N., Belfall B., Duff C., Logan C., Kean V., Thompson M. W., Sylvester J. E., Gorski J. L., Schmickel R. D., Worton R. G. Cloning of the breakpoint of an X;21 translocation associated with Duchenne muscular dystrophy. Nature. 1985 Dec 19;318(6047):672–675. doi: 10.1038/318672a0. [DOI] [PubMed] [Google Scholar]
- Reed K. M., Sites J. W., Jr, Greenbaum I. F. Synapsis, recombination, and meiotic segregation in the mesquite lizard, Sceloporus grammicus, complex. I. Pericentric inversion heteromorphism of the F5 cytotype. Cytogenet Cell Genet. 1992;61(1):40–45. doi: 10.1159/000133366. [DOI] [PubMed] [Google Scholar]
- Rivas F., García-Esquivel L., Rivera H., Jiménez M. E., González R. M., Cantú J. M. Inv(4)(p16q21). A five-generation pedigree with 24 carriers and no recombinants. Clin Genet. 1987 Feb;31(2):97–101. doi: 10.1111/j.1399-0004.1987.tb02776.x. [DOI] [PubMed] [Google Scholar]
- Saadallah N., Hultén M. EM investigations of surface spread synaptonemal complexes in a human male carrier of a pericentric inversion inv(13)(p12q14): the role of heterosynapsis for spermatocyte survival. Ann Hum Genet. 1986 Oct;50(Pt 4):369–383. doi: 10.1111/j.1469-1809.1986.tb01758.x. [DOI] [PubMed] [Google Scholar]
- Schorderet D. F., Friedman C., Disteche C. M. Pericentric inversion of the X chromosome: presentation of a case and review of the literature. Ann Genet. 1991;34(2):98–103. [PubMed] [Google Scholar]
- Sutherland G. R., Gardiner A. J., Carter R. F. Familial pericentric inversion of chromosome 19, inv(19) (p13q13) with a note on genetic counseling of pericentric inversion carriers. Clin Genet. 1976 Jul;10(1):54–59. doi: 10.1111/j.1399-0004.1976.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Trunca C., Opitz J. M. Pericentric inversion of chromosome 14 and the risk of partial duplication of 14q (14q31 leads to 14qter). Am J Med Genet. 1977;1(2):217–228. doi: 10.1002/ajmg.1320010208. [DOI] [PubMed] [Google Scholar]
- Van der Linden A. G., Pearson P. L., Van de Kamp J. J. Cytological assessment of meiotic exchange in a human male with a pericentric inversion of chromosome No. 4. Cytogenet Cell Genet. 1975;14(2):126–139. doi: 10.1159/000130332. [DOI] [PubMed] [Google Scholar]
- Weissenbach J., Gyapay G., Dib C., Vignal A., Morissette J., Millasseau P., Vaysseix G., Lathrop M. A second-generation linkage map of the human genome. Nature. 1992 Oct 29;359(6398):794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]
- de Perdigo A., Gabriel-Robez O., Rumpler Y. Correlation between chromosomal breakpoint positions and synaptic behaviour in human males heterozygous for a pericentric inversion. Hum Genet. 1989 Oct;83(3):274–276. doi: 10.1007/BF00285171. [DOI] [PubMed] [Google Scholar]