Abstract
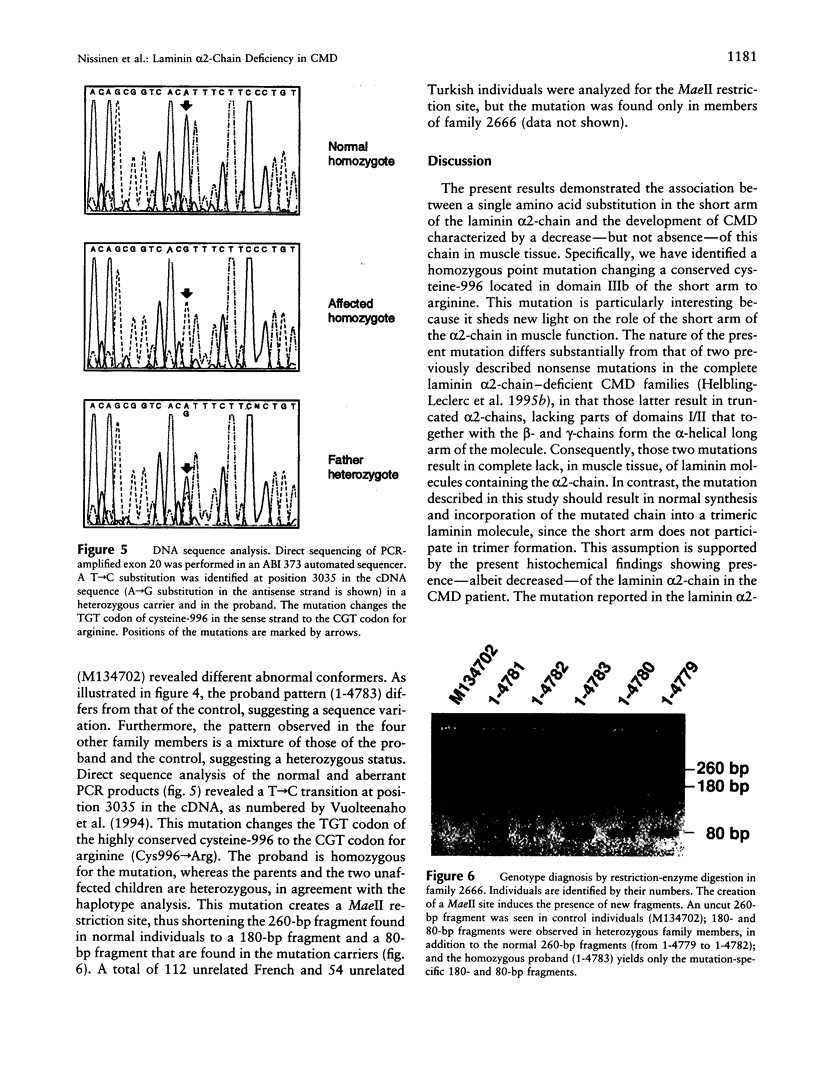
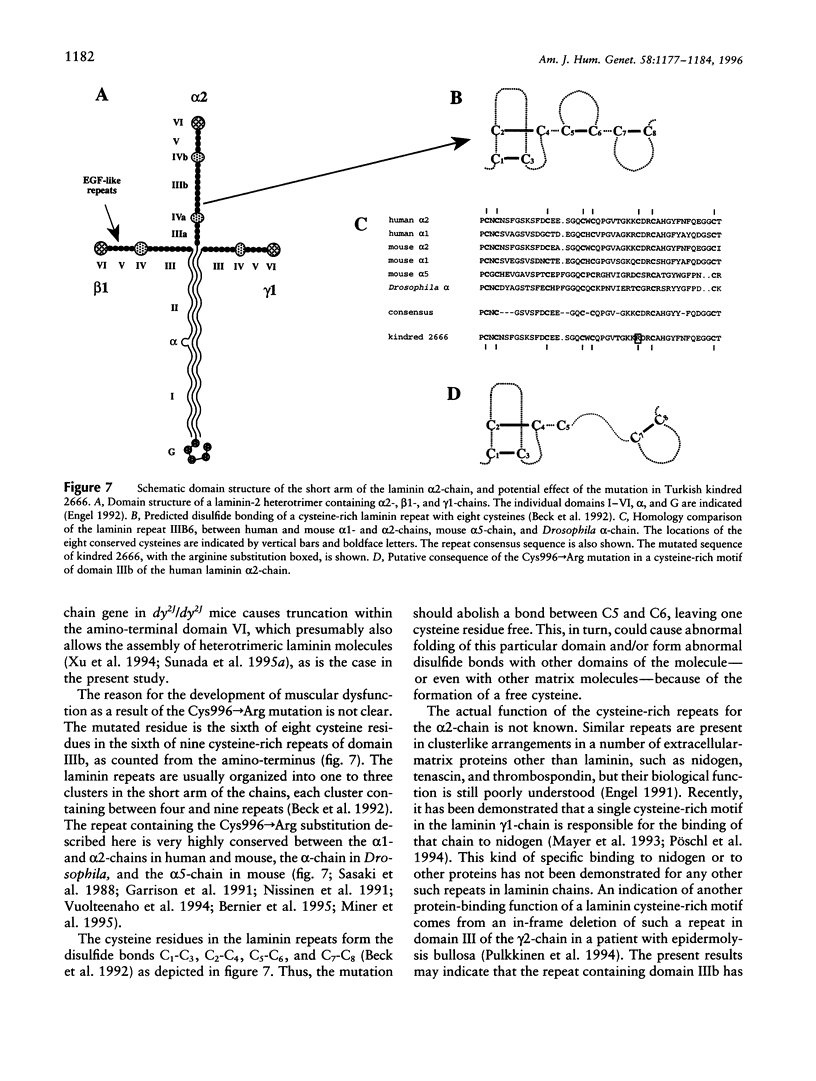
Congenital muscular dystrophies (CMDs) are autosomal recessive muscle disorders of early onset. Approximately half of CMD patients present laminin alpha2-chain (merosin) deficiency in muscle biopsies, and the disease locus has been mapped to the region of the LAMA2 gene (6q22-23) in several families. Recently, two nonsense mutations in the laminin alpha2-chain gene were identified in CMD patients exhibiting complete deficiency of the laminin alpha2-chain in muscle biopsies. However, a subset of CMD patients with linkage to LAMA2 show only partial absence of the laminin alpha2-chain around muscle fibers, by immunocytochemical analysis. In the present study we have identified a homozygous missense mutation in the alpha2-chain gene of a consanguineous Turkish family with partial laminin alpha2-chain deficiency. The T-->C transition at position 3035 in the cDNA sequence results in a Cys996-->Arg substitution. The mutation that affects one of the conserved cysteine-rich repeats in the short arm of the laminin alpha2-chain should result in normal synthesis of the chain and in formation and secretion of a heterotrimeric laminin molecule. Muscular dysfunction is possibly caused either by abnormal disulfide cross-links and folding of the laminin repeat, leading to the disturbance of an as yet unknown binding function of the laminin alpha2-chain and to shorter half-life of the muscle-specific laminin-2 and laminin-4 isoforms, or by increased proteolytic sensitivity, leading to truncation of the short arm.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernier S. M., Utani A., Sugiyama S., Doi T., Polistina C., Yamada Y. Cloning and expression of laminin alpha 2 chain (M-chain) in the mouse. Matrix Biol. 1995 Feb;14(6):447–455. doi: 10.1016/0945-053x(95)90002-0. [DOI] [PubMed] [Google Scholar]
- Burgeson R. E., Chiquet M., Deutzmann R., Ekblom P., Engel J., Kleinman H., Martin G. R., Meneguzzi G., Paulsson M., Sanes J. A new nomenclature for the laminins. Matrix Biol. 1994 Apr;14(3):209–211. doi: 10.1016/0945-053x(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Bönnemann C. G., Modi R., Noguchi S., Mizuno Y., Yoshida M., Gussoni E., McNally E. M., Duggan D. J., Angelini C., Hoffman E. P. Beta-sarcoglycan (A3b) mutations cause autosomal recessive muscular dystrophy with loss of the sarcoglycan complex. Nat Genet. 1995 Nov;11(3):266–273. doi: 10.1038/ng1195-266. [DOI] [PubMed] [Google Scholar]
- Campbell K. P. Three muscular dystrophies: loss of cytoskeleton-extracellular matrix linkage. Cell. 1995 Mar 10;80(5):675–679. doi: 10.1016/0092-8674(95)90344-5. [DOI] [PubMed] [Google Scholar]
- Dib C., Fauré S., Fizames C., Samson D., Drouot N., Vignal A., Millasseau P., Marc S., Hazan J., Seboun E. A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature. 1996 Mar 14;380(6570):152–154. doi: 10.1038/380152a0. [DOI] [PubMed] [Google Scholar]
- Don R. H., Cox P. T., Wainwright B. J., Baker K., Mattick J. S. 'Touchdown' PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 1991 Jul 25;19(14):4008–4008. doi: 10.1093/nar/19.14.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowitz V. 22nd ENMC sponsored workshop on congenital muscular dystrophy held in Baarn, The Netherlands, 14-16 May 1993. Neuromuscul Disord. 1994 Jan;4(1):75–81. doi: 10.1016/0960-8966(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Dubowitz V., Fardeau M. Proceedings of the 27th ENMC sponsored workshop on congenital muscular dystrophy. 22-24 April 1994, The Netherlands. Neuromuscul Disord. 1995 May;5(3):253–258. doi: 10.1016/0960-8966(95)90011-x. [DOI] [PubMed] [Google Scholar]
- Ehrig K., Leivo I., Argraves W. S., Ruoslahti E., Engvall E. Merosin, a tissue-specific basement membrane protein, is a laminin-like protein. Proc Natl Acad Sci U S A. 1990 May;87(9):3264–3268. doi: 10.1073/pnas.87.9.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. Common structural motifs in proteins of the extracellular matrix. Curr Opin Cell Biol. 1991 Oct;3(5):779–785. doi: 10.1016/0955-0674(91)90050-9. [DOI] [PubMed] [Google Scholar]
- Garrison K., MacKrell A. J., Fessler J. H. Drosophila laminin A chain sequence, interspecies comparison, and domain structure of a major carboxyl portion. J Biol Chem. 1991 Dec 5;266(34):22899–22904. [PubMed] [Google Scholar]
- Gyapay G., Morissette J., Vignal A., Dib C., Fizames C., Millasseau P., Marc S., Bernardi G., Lathrop M., Weissenbach J. The 1993-94 Généthon human genetic linkage map. Nat Genet. 1994 Jun;7(2 Spec No):246–339. doi: 10.1038/ng0694supp-246. [DOI] [PubMed] [Google Scholar]
- Hantaï D., Labat-Robert J., Grimaud J. A., Fardeau M. Fibronectin, laminin, type I, III and IV collagens in Duchenne's muscular dystrophy, congenital muscular dystrophies and congenital myopathies: an immunocytochemical study. Connect Tissue Res. 1985;13(4):273–281. doi: 10.3109/03008208509152408. [DOI] [PubMed] [Google Scholar]
- Helbling-Leclerc A., Topaloglu H., Tomé F. M., Sewry C., Gyapay G., Naom I., Muntoni F., Dubowitz V., Barois A., Estournet B. Readjusting the localization of merosin (laminin alpha 2-chain) deficient congenital muscular dystrophy locus on chromosome 6q2. C R Acad Sci III. 1995 Dec;318(12):1245–1252. [PubMed] [Google Scholar]
- Helbling-Leclerc A., Zhang X., Topaloglu H., Cruaud C., Tesson F., Weissenbach J., Tomé F. M., Schwartz K., Fardeau M., Tryggvason K. Mutations in the laminin alpha 2-chain gene (LAMA2) cause merosin-deficient congenital muscular dystrophy. Nat Genet. 1995 Oct;11(2):216–218. doi: 10.1038/ng1095-216. [DOI] [PubMed] [Google Scholar]
- Hillaire D., Leclerc A., Fauré S., Topaloglu H., Chiannilkulchaï N., Guicheney P., Grinas L., Legos P., Philpot J., Evangelista T. Localization of merosin-negative congenital muscular dystrophy to chromosome 6q2 by homozygosity mapping. Hum Mol Genet. 1994 Sep;3(9):1657–1661. doi: 10.1093/hmg/3.9.1657. [DOI] [PubMed] [Google Scholar]
- Lander E. S., Botstein D. Homozygosity mapping: a way to map human recessive traits with the DNA of inbred children. Science. 1987 Jun 19;236(4808):1567–1570. doi: 10.1126/science.2884728. [DOI] [PubMed] [Google Scholar]
- Leivo I., Engvall E. Merosin, a protein specific for basement membranes of Schwann cells, striated muscle, and trophoblast, is expressed late in nerve and muscle development. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1544–1548. doi: 10.1073/pnas.85.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U., Nischt R., Pöschl E., Mann K., Fukuda K., Gerl M., Yamada Y., Timpl R. A single EGF-like motif of laminin is responsible for high affinity nidogen binding. EMBO J. 1993 May;12(5):1879–1885. doi: 10.1002/j.1460-2075.1993.tb05836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner J. H., Lewis R. M., Sanes J. R. Molecular cloning of a novel laminin chain, alpha 5, and widespread expression in adult mouse tissues. J Biol Chem. 1995 Dec 1;270(48):28523–28526. doi: 10.1074/jbc.270.48.28523. [DOI] [PubMed] [Google Scholar]
- Monaco A. P., Neve R. L., Colletti-Feener C., Bertelson C. J., Kurnit D. M., Kunkel L. M. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature. 1986 Oct 16;323(6089):646–650. doi: 10.1038/323646a0. [DOI] [PubMed] [Google Scholar]
- Nissinen M., Vuolteenaho R., Boot-Handford R., Kallunki P., Tryggvason K. Primary structure of the human laminin A chain. Limited expression in human tissues. Biochem J. 1991 Jun 1;276(Pt 2):369–379. doi: 10.1042/bj2760369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi S., McNally E. M., Ben Othmane K., Hagiwara Y., Mizuno Y., Yoshida M., Yamamoto H., Bönnemann C. G., Gussoni E., Denton P. H. Mutations in the dystrophin-associated protein gamma-sarcoglycan in chromosome 13 muscular dystrophy. Science. 1995 Nov 3;270(5237):819–822. doi: 10.1126/science.270.5237.819. [DOI] [PubMed] [Google Scholar]
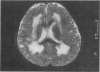
- Philpot J., Topaloglu H., Pennock J., Dubowitz V. Familial concordance of brain magnetic resonance imaging changes in congenital muscular dystrophy. Neuromuscul Disord. 1995 May;5(3):227–231. doi: 10.1016/0960-8966(94)00047-d. [DOI] [PubMed] [Google Scholar]
- Piccolo F., Roberds S. L., Jeanpierre M., Leturcq F., Azibi K., Beldjord C., Carrié A., Récan D., Chaouch M., Reghis A. Primary adhalinopathy: a common cause of autosomal recessive muscular dystrophy of variable severity. Nat Genet. 1995 Jun;10(2):243–245. doi: 10.1038/ng0695-243. [DOI] [PubMed] [Google Scholar]
- Pulkkinen L., Christiano A. M., Airenne T., Haakana H., Tryggvason K., Uitto J. Mutations in the gamma 2 chain gene (LAMC2) of kalinin/laminin 5 in the junctional forms of epidermolysis bullosa. Nat Genet. 1994 Mar;6(3):293–297. doi: 10.1038/ng0394-293. [DOI] [PubMed] [Google Scholar]
- Pöschl E., Fox J. W., Block D., Mayer U., Timpl R. Two non-contiguous regions contribute to nidogen binding to a single EGF-like motif of the laminin gamma 1 chain. EMBO J. 1994 Aug 15;13(16):3741–3747. doi: 10.1002/j.1460-2075.1994.tb06683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberds S. L., Leturcq F., Allamand V., Piccolo F., Jeanpierre M., Anderson R. D., Lim L. E., Lee J. C., Tomé F. M., Romero N. B. Missense mutations in the adhalin gene linked to autosomal recessive muscular dystrophy. Cell. 1994 Aug 26;78(4):625–633. doi: 10.1016/0092-8674(94)90527-4. [DOI] [PubMed] [Google Scholar]
- Sasaki M., Kleinman H. K., Huber H., Deutzmann R., Yamada Y. Laminin, a multidomain protein. The A chain has a unique globular domain and homology with the basement membrane proteoglycan and the laminin B chains. J Biol Chem. 1988 Nov 15;263(32):16536–16544. [PubMed] [Google Scholar]
- Stephens H. R., Duance V. C., Dunn M. J., Bailey A. J., Dubowitz V. Collagen types in neuromuscular diseases. J Neurol Sci. 1982 Jan;53(1):45–62. doi: 10.1016/0022-510x(82)90079-x. [DOI] [PubMed] [Google Scholar]
- Sunada Y., Bernier S. M., Utani A., Yamada Y., Campbell K. P. Identification of a novel mutant transcript of laminin alpha 2 chain gene responsible for muscular dystrophy and dysmyelination in dy2J mice. Hum Mol Genet. 1995 Jun;4(6):1055–1061. doi: 10.1093/hmg/4.6.1055. [DOI] [PubMed] [Google Scholar]
- Sunada Y., Edgar T. S., Lotz B. P., Rust R. S., Campbell K. P. Merosin-negative congenital muscular dystrophy associated with extensive brain abnormalities. Neurology. 1995 Nov;45(11):2084–2089. doi: 10.1212/wnl.45.11.2084. [DOI] [PubMed] [Google Scholar]
- Tomé F. M., Evangelista T., Leclerc A., Sunada Y., Manole E., Estournet B., Barois A., Campbell K. P., Fardeau M. Congenital muscular dystrophy with merosin deficiency. C R Acad Sci III. 1994 Apr;317(4):351–357. [PubMed] [Google Scholar]
- Tryggvason K. The laminin family. Curr Opin Cell Biol. 1993 Oct;5(5):877–882. doi: 10.1016/0955-0674(93)90038-r. [DOI] [PubMed] [Google Scholar]
- Vuolteenaho R., Nissinen M., Sainio K., Byers M., Eddy R., Hirvonen H., Shows T. B., Sariola H., Engvall E., Tryggvason K. Human laminin M chain (merosin): complete primary structure, chromosomal assignment, and expression of the M and A chain in human fetal tissues. J Cell Biol. 1994 Feb;124(3):381–394. doi: 10.1083/jcb.124.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worton R. Muscular dystrophies: diseases of the dystrophin-glycoprotein complex. Science. 1995 Nov 3;270(5237):755–756. doi: 10.1126/science.270.5237.755. [DOI] [PubMed] [Google Scholar]
- Xu H., Wu X. R., Wewer U. M., Engvall E. Murine muscular dystrophy caused by a mutation in the laminin alpha 2 (Lama2) gene. Nat Genet. 1994 Nov;8(3):297–302. doi: 10.1038/ng1194-297. [DOI] [PubMed] [Google Scholar]