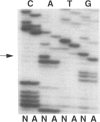
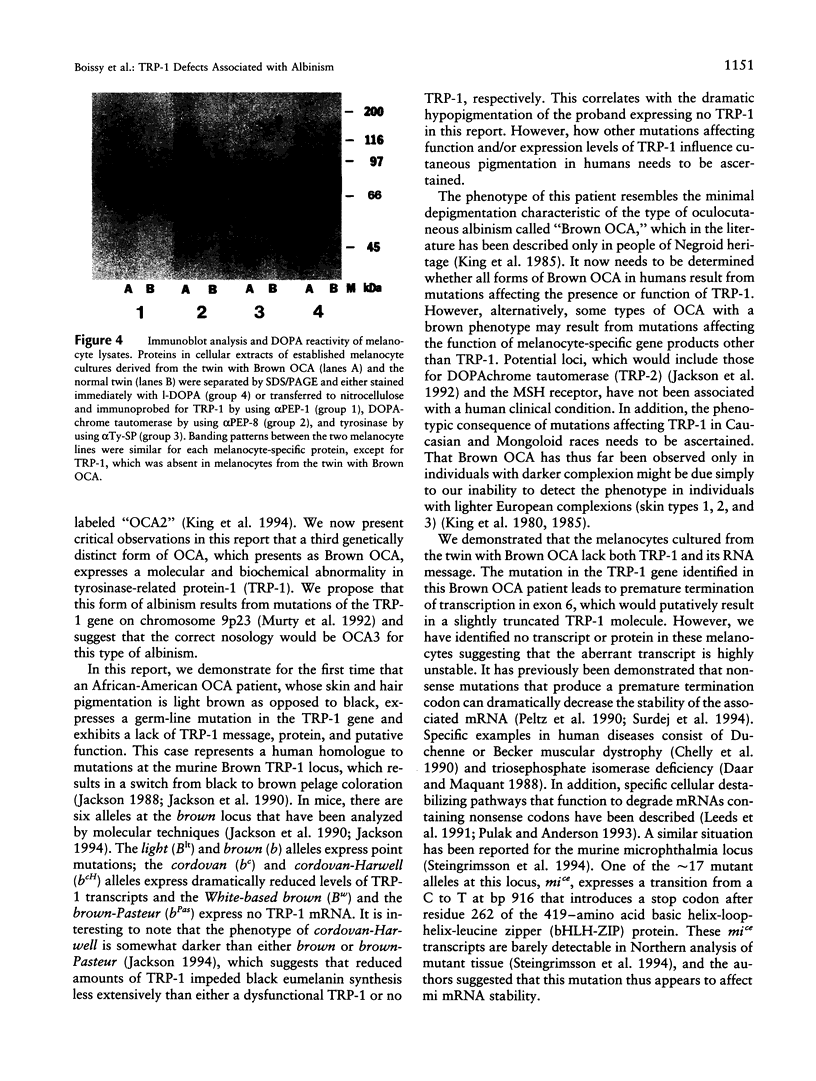
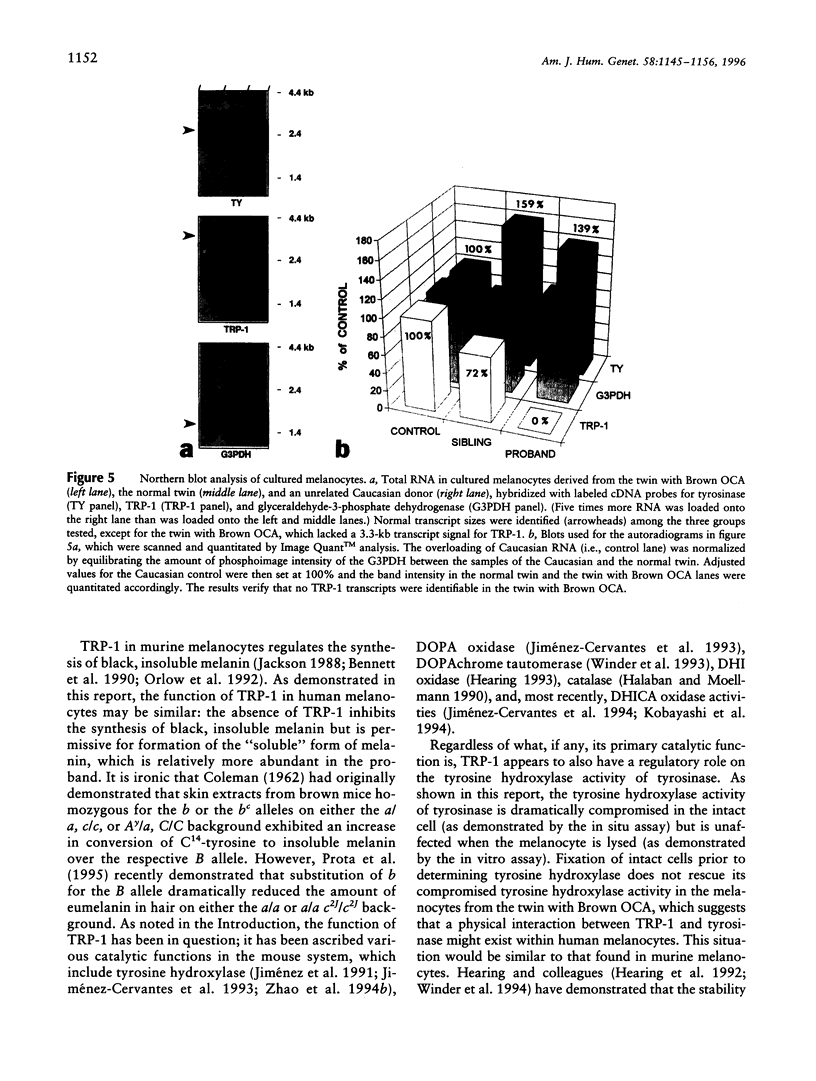
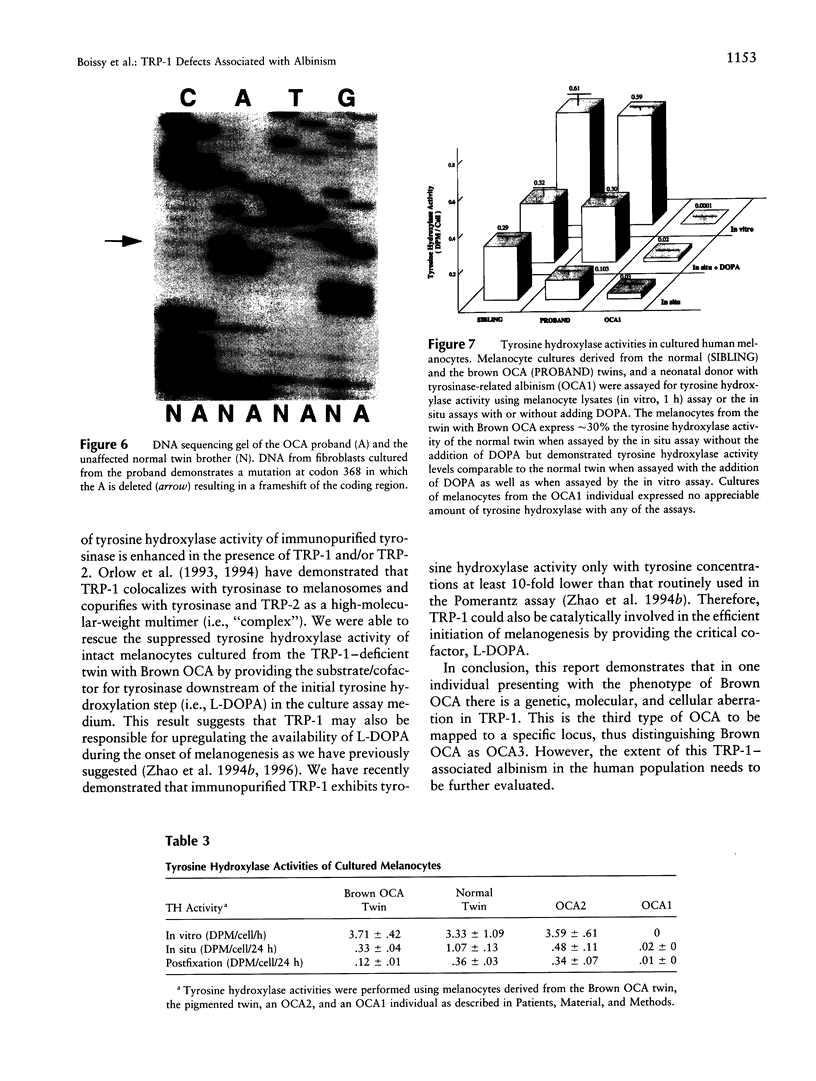
Abstract
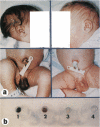
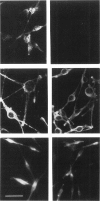
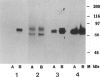
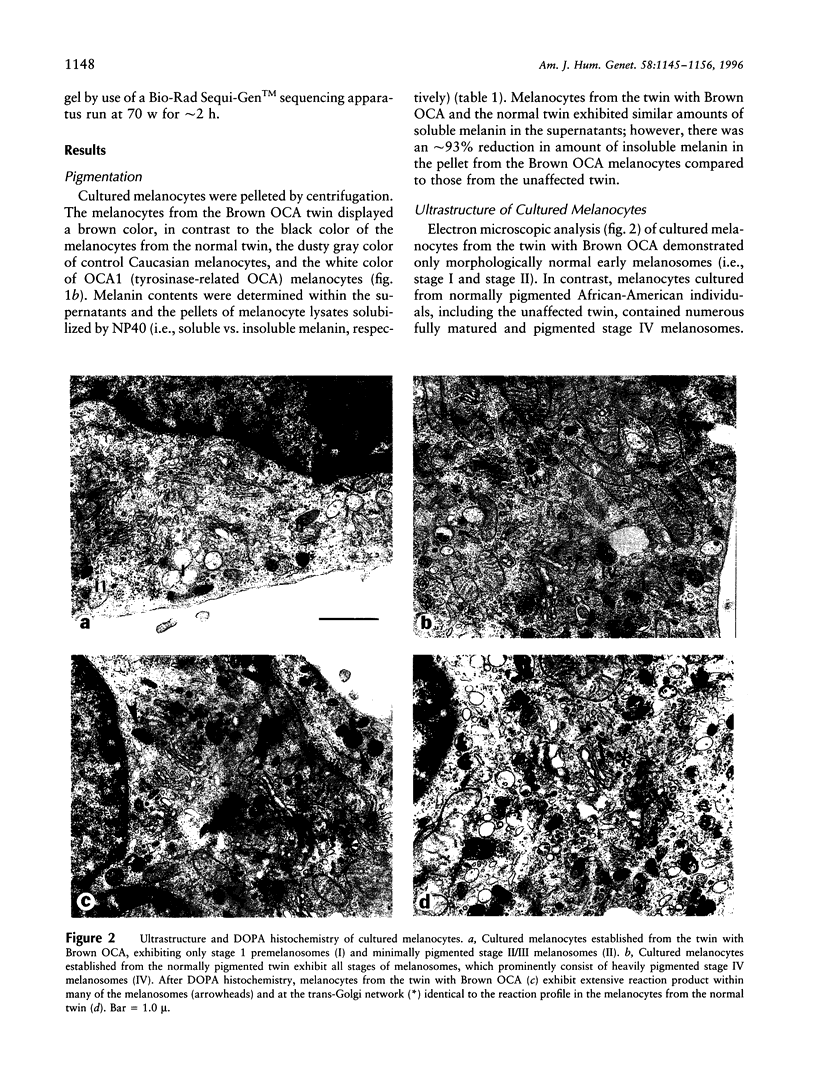
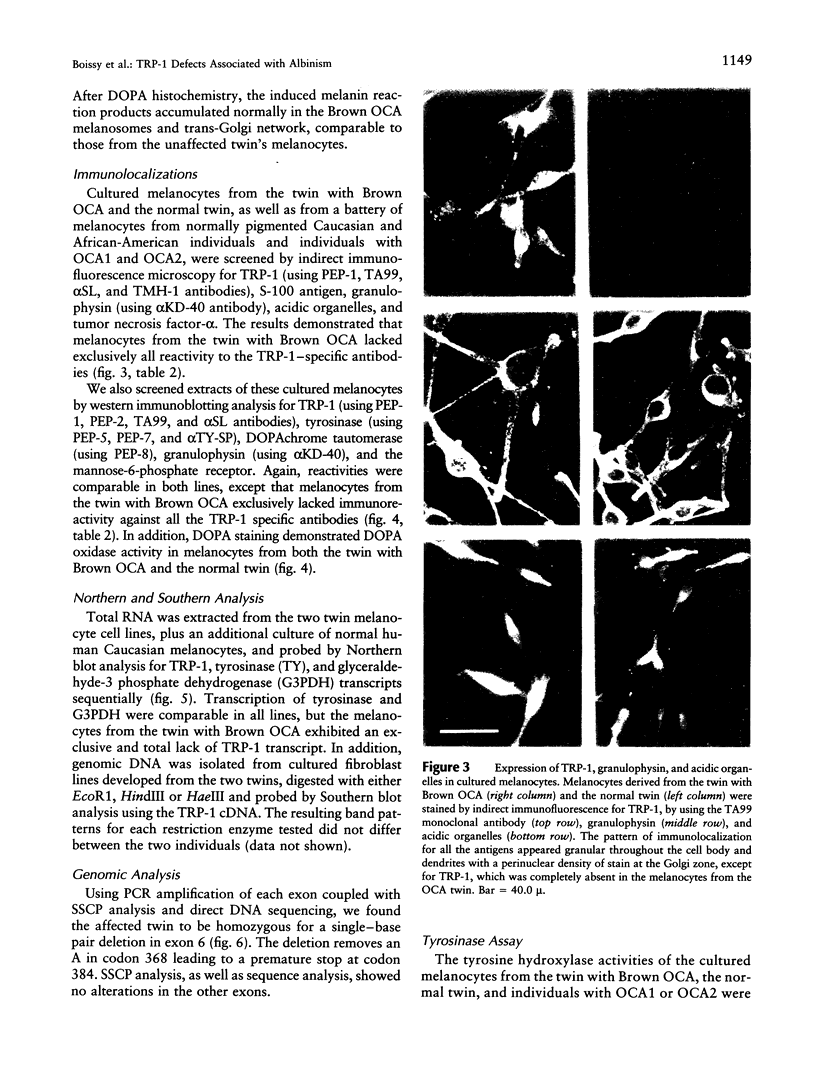
Most types of human oculocutaneous albinism (OCA) result from mutations in the gene for tyrosinase (OCA1) or the P protein (OCA2), although other types of OCA have been described but have not been mapped to specific loci. Melanocytes were cultured from an African-American with OCA, who exhibited the phenotype of Brown OCA, and his normal fraternal twin. Melanocytes cultured from the patient with OCA and the normal twin appeared brown versus black, respectively. Melanocytes from both the patient with OCA and the normal twin demonstrated equal amounts of NP-40-soluble melanin; however, melanocytes from the patient with OCA contained only 7% of the amount of insoluble melanin found from the normal twin. Tyrosinase- related protein-1 (TRP-1) was not detected in the OCA melanocytes by use of various anti-TRP-1 probes. Furthermore, transcripts for TRP-1 were absent in cultured OCA melanocytes. The affected twin was homozygous for a single-bp deletion in exon 6, removing an A in codon 368 and leading to a premature stop at codon 384. Tyrosine hydroxylase activity of the OCA melanocytes was comparable to controls when assayed in cell lysates but was only 30% of controls when assayed in intact cells. We conclude that this mutation of the human TRP-1 gene affects its interaction with tyrosinase, resulting in dysregulation of tyrosinase activity, promotes the synthesis of brown versus black melanin, and is responsible for a third genetic type of OCA in humans, which we classify as "OCA3."
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Falck J. R., Goldstein J. L., Brown M. S. Visualization of acidic organelles in intact cells by electron microscopy. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4838–4842. doi: 10.1073/pnas.81.15.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin L. M., Boissy R. E. Mammalian tyrosinase-related protein-1 is recognized by autoantibodies from vitiliginous Smyth chickens. An avian model for human vitiligo. Am J Pathol. 1995 Jun;146(6):1529–1541. [PMC free article] [PubMed] [Google Scholar]
- Barton D. E., Kwon B. S., Francke U. Human tyrosinase gene, mapped to chromosome 11 (q14----q21), defines second region of homology with mouse chromosome 7. Genomics. 1988 Jul;3(1):17–24. doi: 10.1016/0888-7543(88)90153-x. [DOI] [PubMed] [Google Scholar]
- Bennett D. C., Huszar D., Laipis P. J., Jaenisch R., Jackson I. J. Phenotypic rescue of mutant brown melanocytes by a retrovirus carrying a wild-type tyrosinase-related protein gene. Development. 1990 Oct;110(2):471–475. doi: 10.1242/dev.110.2.471. [DOI] [PubMed] [Google Scholar]
- Boissy R. E., Liu Y. Y., Medrano E. E., Nordlund J. J. Structural aberration of the rough endoplasmic reticulum and melanosome compartmentalization in long-term cultures of melanocytes from vitiligo patients. J Invest Dermatol. 1991 Sep;97(3):395–404. doi: 10.1111/1523-1747.ep12480976. [DOI] [PubMed] [Google Scholar]
- Bouchard B., Fuller B. B., Vijayasaradhi S., Houghton A. N. Induction of pigmentation in mouse fibroblasts by expression of human tyrosinase cDNA. J Exp Med. 1989 Jun 1;169(6):2029–2042. doi: 10.1084/jem.169.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLEMAN D. L. Effect of genic substitution on the incorporation of tyrosine into the melanin of mouse skin. Arch Biochem Biophys. 1962 Mar;96:562–568. doi: 10.1016/0003-9861(62)90337-5. [DOI] [PubMed] [Google Scholar]
- Chelly J., Gilgenkrantz H., Lambert M., Hamard G., Chafey P., Récan D., Katz P., de la Chapelle A., Koenig M., Ginjaar I. B. Effect of dystrophin gene deletions on mRNA levels and processing in Duchenne and Becker muscular dystrophies. Cell. 1990 Dec 21;63(6):1239–1248. doi: 10.1016/0092-8674(90)90419-f. [DOI] [PubMed] [Google Scholar]
- Chintamaneni C. D., Halaban R., Kobayashi Y., Witkop C. J., Jr, Kwon B. S. A single base insertion in the putative transmembrane domain of the tyrosinase gene as a cause for tyrosinase-negative oculocutaneous albinism. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5272–5276. doi: 10.1073/pnas.88.12.5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintamaneni C. D., Ramsay M., Colman M. A., Fox M. F., Pickard R. T., Kwon B. S. Mapping the human CAS2 gene, the homologue of the mouse brown (b) locus, to human chromosome 9p22-pter. Biochem Biophys Res Commun. 1991 Jul 15;178(1):227–235. doi: 10.1016/0006-291x(91)91803-k. [DOI] [PubMed] [Google Scholar]
- Cochran A. J., Wen D. R., Herschman H. R., Gaynor R. B. Detection of S-100 protein as an aid to the identification of melanocytic tumors. Int J Cancer. 1982 Sep 15;30(3):295–297. doi: 10.1002/ijc.2910300307. [DOI] [PubMed] [Google Scholar]
- Cohen T., Muller R. M., Tomita Y., Shibahara S. Nucleotide sequence of the cDNA encoding human tyrosinase-related protein. Nucleic Acids Res. 1990 May 11;18(9):2807–2808. doi: 10.1093/nar/18.9.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daar I. O., Maquat L. E. Premature translation termination mediates triosephosphate isomerase mRNA degradation. Mol Cell Biol. 1988 Feb;8(2):802–813. doi: 10.1128/mcb.8.2.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham-Pierre D., Gardner J. M., Nakatsu Y., King R. A., Francke U., Ching A., Aquaron R., del Marmol V., Brilliant M. H. African origin of an intragenic deletion of the human P gene in tyrosinase positive oculocutaneous albinism. Nat Genet. 1994 Jun;7(2):176–179. doi: 10.1038/ng0694-176. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gardner J. M., Nakatsu Y., Gondo Y., Lee S., Lyon M. F., King R. A., Brilliant M. H. The mouse pink-eyed dilution gene: association with human Prader-Willi and Angelman syndromes. Science. 1992 Aug 21;257(5073):1121–1124. doi: 10.1126/science.257.5073.1121. [DOI] [PubMed] [Google Scholar]
- Gerrard J. M., Lint D., Sims P. J., Wiedmer T., Fugate R. D., McMillan E., Robertson C., Israels S. J. Identification of a platelet dense granule membrane protein that is deficient in a patient with the Hermansky-Pudlak syndrome. Blood. 1991 Jan 1;77(1):101–112. [PubMed] [Google Scholar]
- Giebel L. B., Tripathi R. K., King R. A., Spritz R. A. A tyrosinase gene missense mutation in temperature-sensitive type I oculocutaneous albinism. A human homologue to the Siamese cat and the Himalayan mouse. J Clin Invest. 1991 Mar;87(3):1119–1122. doi: 10.1172/JCI115075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebel L. B., Tripathi R. K., Strunk K. M., Hanifin J. M., Jackson C. E., King R. A., Spritz R. A. Tyrosinase gene mutations associated with type IB ("yellow") oculocutaneous albinism. Am J Hum Genet. 1991 Jun;48(6):1159–1167. [PMC free article] [PubMed] [Google Scholar]
- Halaban R., Moellmann G. Murine and human b locus pigmentation genes encode a glycoprotein (gp75) with catalase activity. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4809–4813. doi: 10.1073/pnas.87.12.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaban R., Pomerantz S. H., Marshall S., Lambert D. T., Lerner A. B. Regulation of tyrosinase in human melanocytes grown in culture. J Cell Biol. 1983 Aug;97(2):480–488. doi: 10.1083/jcb.97.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing V. J., Tsukamoto K., Urabe K., Kameyama K., Montague P. M., Jackson I. J. Functional properties of cloned melanogenic proteins. Pigment Cell Res. 1992 Nov;5(5 Pt 2):264–270. doi: 10.1111/j.1600-0749.1992.tb00547.x. [DOI] [PubMed] [Google Scholar]
- Hearing V. J. Unraveling the melanocyte. Am J Hum Genet. 1993 Jan;52(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Jackson I. J. A cDNA encoding tyrosinase-related protein maps to the brown locus in mouse. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4392–4396. doi: 10.1073/pnas.85.12.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson I. J., Chambers D. M., Tsukamoto K., Copeland N. G., Gilbert D. J., Jenkins N. A., Hearing V. A second tyrosinase-related protein, TRP-2, maps to and is mutated at the mouse slaty locus. EMBO J. 1992 Feb;11(2):527–535. doi: 10.1002/j.1460-2075.1992.tb05083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson I. J., Chambers D., Rinchik E. M., Bennett D. C. Characterization of TRP-1 mRNA levels in dominant and recessive mutations at the mouse brown (b) locus. Genetics. 1990 Oct;126(2):451–459. doi: 10.1093/genetics/126.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson I. J. Molecular and developmental genetics of mouse coat color. Annu Rev Genet. 1994;28:189–217. doi: 10.1146/annurev.ge.28.120194.001201. [DOI] [PubMed] [Google Scholar]
- Jimenez-Cervantes C., Garcia-Borron J. C., Valverde P., Solano F., Lozano J. A. Tyrosinase isoenzymes in mammalian melanocytes. 1. Biochemical characterization of two melanosomal tyrosinases from B16 mouse melanoma. Eur J Biochem. 1993 Oct 15;217(2):549–556. doi: 10.1111/j.1432-1033.1993.tb18276.x. [DOI] [PubMed] [Google Scholar]
- Jiménez M., Maloy W. L., Hearing V. J. Specific identification of an authentic clone for mammalian tyrosinase. J Biol Chem. 1989 Feb 25;264(6):3397–3403. [PubMed] [Google Scholar]
- Jiménez M., Tsukamoto K., Hearing V. J. Tyrosinases from two different loci are expressed by normal and by transformed melanocytes. J Biol Chem. 1991 Jan 15;266(2):1147–1156. [PubMed] [Google Scholar]
- King R. A., Creel D., Cervenka J., Okoro A. N., Witkop C. J. Albinism in Nigeria with delineation of new recessive oculocutaneous type. Clin Genet. 1980 Apr;17(4):259–270. doi: 10.1111/j.1399-0004.1980.tb00145.x. [DOI] [PubMed] [Google Scholar]
- King R. A., Lewis R. A., Townsend D., Zelickson A., Olds D. P., Brumbaugh J. Brown oculocutaneous albinism. Clinical, ophthalmological, and biochemical characterization. Ophthalmology. 1985 Nov;92(11):1496–1505. doi: 10.1016/s0161-6420(85)33832-0. [DOI] [PubMed] [Google Scholar]
- King R. A., Mentink M. M., Oetting W. S. Non-random distribution of missense mutations within the human tyrosinase gene in type I (tyrosinase-related) oculocutaneous albinism. Mol Biol Med. 1991 Feb;8(1):19–29. [PubMed] [Google Scholar]
- King R. A., Rich S. S. Segregation analysis of brown oculocutaneous albinism. Clin Genet. 1986 Jun;29(6):496–501. doi: 10.1111/j.1399-0004.1986.tb00550.x. [DOI] [PubMed] [Google Scholar]
- King R. A., Witkop C. J., Jr Hairbulb tyrosinase activity in oculocutaneous albinism. Nature. 1976 Sep 2;263(5572):69–71. doi: 10.1038/263069a0. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Urabe K., Winder A., Jiménez-Cervantes C., Imokawa G., Brewington T., Solano F., García-Borrón J. C., Hearing V. J. Tyrosinase related protein 1 (TRP1) functions as a DHICA oxidase in melanin biosynthesis. EMBO J. 1994 Dec 15;13(24):5818–5825. doi: 10.1002/j.1460-2075.1994.tb06925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. T., Nicholls R. D., Bundey S., Laxova R., Musarella M., Spritz R. A. Mutations of the P gene in oculocutaneous albinism, ocular albinism, and Prader-Willi syndrome plus albinism. N Engl J Med. 1994 Feb 24;330(8):529–534. doi: 10.1056/NEJM199402243300803. [DOI] [PubMed] [Google Scholar]
- Leeds P., Peltz S. W., Jacobson A., Culbertson M. R. The product of the yeast UPF1 gene is required for rapid turnover of mRNAs containing a premature translational termination codon. Genes Dev. 1991 Dec;5(12A):2303–2314. doi: 10.1101/gad.5.12a.2303. [DOI] [PubMed] [Google Scholar]
- Murty V. V., Bouchard B., Mathew S., Vijayasaradhi S., Houghton A. N. Assignment of the human TYRP (brown) locus to chromosome region 9p23 by nonradioactive in situ hybridization. Genomics. 1992 May;13(1):227–229. doi: 10.1016/0888-7543(92)90228-k. [DOI] [PubMed] [Google Scholar]
- Oetting W. S., Fryer J. P., Oofuji Y., Middendorf L. R., Brumbaugh J. A., Summers C. G., King R. A. Analysis of tyrosinase gene mutations using direct automated infrared fluorescence DNA sequencing of amplified exons. Electrophoresis. 1994 Feb;15(2):159–164. doi: 10.1002/elps.1150150127. [DOI] [PubMed] [Google Scholar]
- Orlow S. J., Boissy R. E., Moran D. J., Pifko-Hirst S. Subcellular distribution of tyrosinase and tyrosinase-related protein-1: implications for melanosomal biogenesis. J Invest Dermatol. 1993 Jan;100(1):55–64. doi: 10.1111/1523-1747.ep12354138. [DOI] [PubMed] [Google Scholar]
- Orlow S. J., Osber M. P., Pawelek J. M. Synthesis and characterization of melanins from dihydroxyindole-2-carboxylic acid and dihydroxyindole. Pigment Cell Res. 1992 Sep;5(3):113–121. doi: 10.1111/j.1600-0749.1992.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Orlow S. J., Zhou B. K., Chakraborty A. K., Drucker M., Pifko-Hirst S., Pawelek J. M. High-molecular-weight forms of tyrosinase and the tyrosinase-related proteins: evidence for a melanogenic complex. J Invest Dermatol. 1994 Aug;103(2):196–201. doi: 10.1111/1523-1747.ep12392743. [DOI] [PubMed] [Google Scholar]
- Oshima A., Nolan C. M., Kyle J. W., Grubb J. H., Sly W. S. The human cation-independent mannose 6-phosphate receptor. Cloning and sequence of the full-length cDNA and expression of functional receptor in COS cells. J Biol Chem. 1988 Feb 15;263(5):2553–2562. [PubMed] [Google Scholar]
- Peltz S. W., Brewer G., Bernstein P., Hart P. A., Ross J. Regulation of mRNA turnover in eukaryotic cells. Crit Rev Eukaryot Gene Expr. 1991;1(2):99–126. [PubMed] [Google Scholar]
- Prota G., Lamoreux M. L., Muller J., Kobayashi T., Napolitano A., Vincensi M. R., Sakai C., Hearing V. J. Comparative analysis of melanins and melanosomes produced by various coat color mutants. Pigment Cell Res. 1995 Jun;8(3):153–163. doi: 10.1111/j.1600-0749.1995.tb00657.x. [DOI] [PubMed] [Google Scholar]
- Pulak R., Anderson P. mRNA surveillance by the Caenorhabditis elegans smg genes. Genes Dev. 1993 Oct;7(10):1885–1897. doi: 10.1101/gad.7.10.1885. [DOI] [PubMed] [Google Scholar]
- Ramsay M., Colman M. A., Stevens G., Zwane E., Kromberg J., Farrall M., Jenkins T. The tyrosinase-positive oculocutaneous albinism locus maps to chromosome 15q11.2-q12. Am J Hum Genet. 1992 Oct;51(4):879–884. [PMC free article] [PubMed] [Google Scholar]
- Rinchik E. M., Bultman S. J., Horsthemke B., Lee S. T., Strunk K. M., Spritz R. A., Avidano K. M., Jong M. T., Nicholls R. D. A gene for the mouse pink-eyed dilution locus and for human type II oculocutaneous albinism. Nature. 1993 Jan 7;361(6407):72–76. doi: 10.1038/361072a0. [DOI] [PubMed] [Google Scholar]
- Shibahara S., Tomita Y., Sakakura T., Nager C., Chaudhuri B., Müller R. Cloning and expression of cDNA encoding mouse tyrosinase. Nucleic Acids Res. 1986 Mar 25;14(6):2413–2427. doi: 10.1093/nar/14.6.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steingrímsson E., Moore K. J., Lamoreux M. L., Ferré-D'Amaré A. R., Burley S. K., Zimring D. C., Skow L. C., Hodgkinson C. A., Arnheiter H., Copeland N. G. Molecular basis of mouse microphthalmia (mi) mutations helps explain their developmental and phenotypic consequences. Nat Genet. 1994 Nov;8(3):256–263. doi: 10.1038/ng1194-256. [DOI] [PubMed] [Google Scholar]
- Surdej P., Riedl A., Jacobs-Lorena M. Regulation of mRNA stability in development. Annu Rev Genet. 1994;28:263–282. doi: 10.1146/annurev.ge.28.120194.001403. [DOI] [PubMed] [Google Scholar]
- Thomson T. M., Mattes M. J., Roux L., Old L. J., Lloyd K. O. Pigmentation-associated glycoprotein of human melanomas and melanocytes: definition with a mouse monoclonal antibody. J Invest Dermatol. 1985 Aug;85(2):169–174. doi: 10.1111/1523-1747.ep12276608. [DOI] [PubMed] [Google Scholar]
- Tomita Y., Montague P. M., Hearing V. J. Anti-T4-tyrosinase monoclonal antibodies--specific markers for pigmented melanocytes. J Invest Dermatol. 1985 Nov;85(5):426–430. doi: 10.1111/1523-1747.ep12277121. [DOI] [PubMed] [Google Scholar]
- Tomita Y., Takeda A., Okinaga S., Tagami H., Shibahara S. Human oculocutaneous albinism caused by single base insertion in the tyrosinase gene. Biochem Biophys Res Commun. 1989 Nov 15;164(3):990–996. doi: 10.1016/0006-291x(89)91767-1. [DOI] [PubMed] [Google Scholar]
- Tsukamoto K., Jackson I. J., Urabe K., Montague P. M., Hearing V. J. A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO J. 1992 Feb;11(2):519–526. doi: 10.1002/j.1460-2075.1992.tb05082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart A. Human tyrosinase-like protein (TYRL) carboxy terminus: closer homology with the mouse protein than previously reported. Nucleic Acids Res. 1991 Oct 25;19(20):5803–5803. doi: 10.1093/nar/19.20.5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder A., Kobayashi T., Tsukamoto K., Urabe K., Aroca P., Kameyama K., Hearing V. J. The tyrosinase gene family--interactions of melanogenic proteins to regulate melanogenesis. Cell Mol Biol Res. 1994;40(7-8):613–626. [PubMed] [Google Scholar]
- Witkop C. J., Jr Albinism. Adv Hum Genet. 1971;2:61–142. [PubMed] [Google Scholar]
- Zhao H., Boissy R. E. Distinguishing between the catalytic potential and apparent expression of tyrosinase activities. Am J Med Sci. 1994 Dec;308(6):322–330. doi: 10.1097/00000441-199412000-00003. [DOI] [PubMed] [Google Scholar]
- Zhao H., Boissy Y. L., Abdel-Malek Z., King R. A., Nordlund J. J., Boissy R. E. On the analysis of the pathophysiology of Chediak-Higashi syndrome. Defects expressed by cultured melanocytes. Lab Invest. 1994 Jul;71(1):25–34. [PubMed] [Google Scholar]
- Zhao H., Eling D. J., Medrano E. E., Boissy R. E. Retroviral infection with human tyrosinase-related protein-1 (TRP-1) cDNA upregulates tyrosinase activity and melanin synthesis in a TRP-1-deficient melanoma cell line. J Invest Dermatol. 1996 Apr;106(4):744–752. doi: 10.1111/1523-1747.ep12345799. [DOI] [PubMed] [Google Scholar]
- Zhao H., Zhao Y., Nordlund J. J., Boissy R. E. Human TRP-1 has tyrosine hydroxylase but no dopa oxidase activity. Pigment Cell Res. 1994 Jun;7(3):131–140. doi: 10.1111/j.1600-0749.1994.tb00040.x. [DOI] [PubMed] [Google Scholar]