Abstract
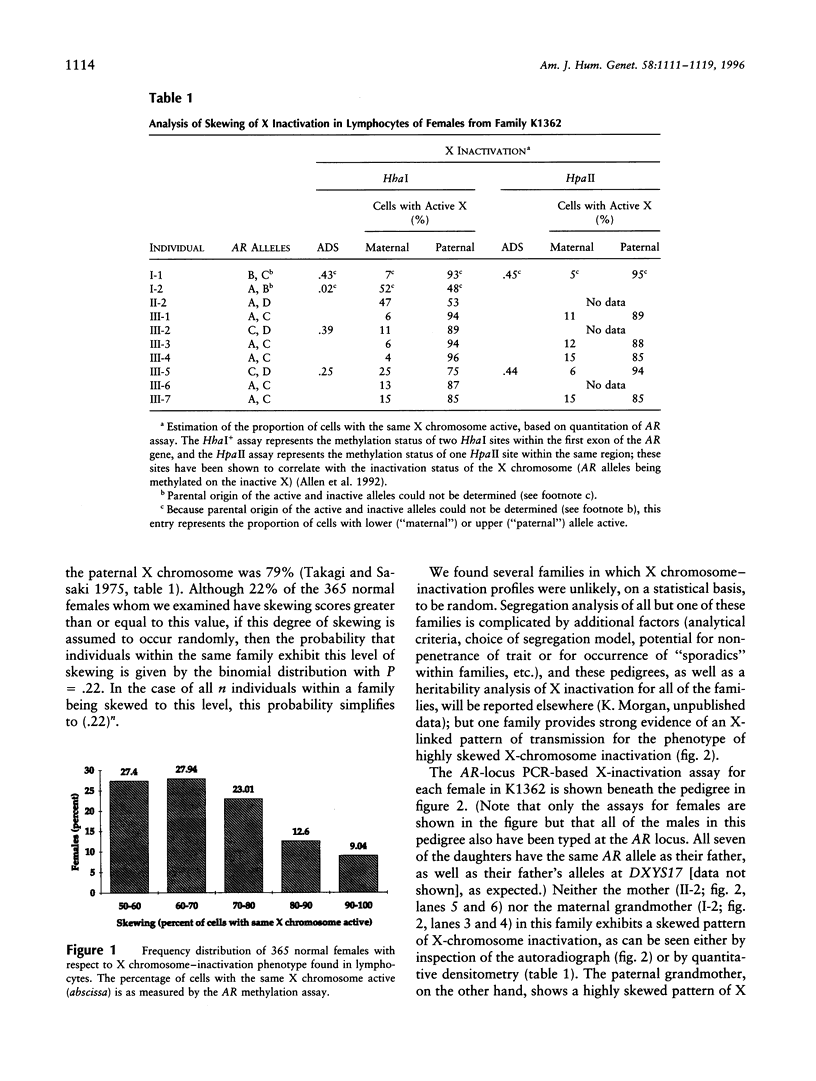
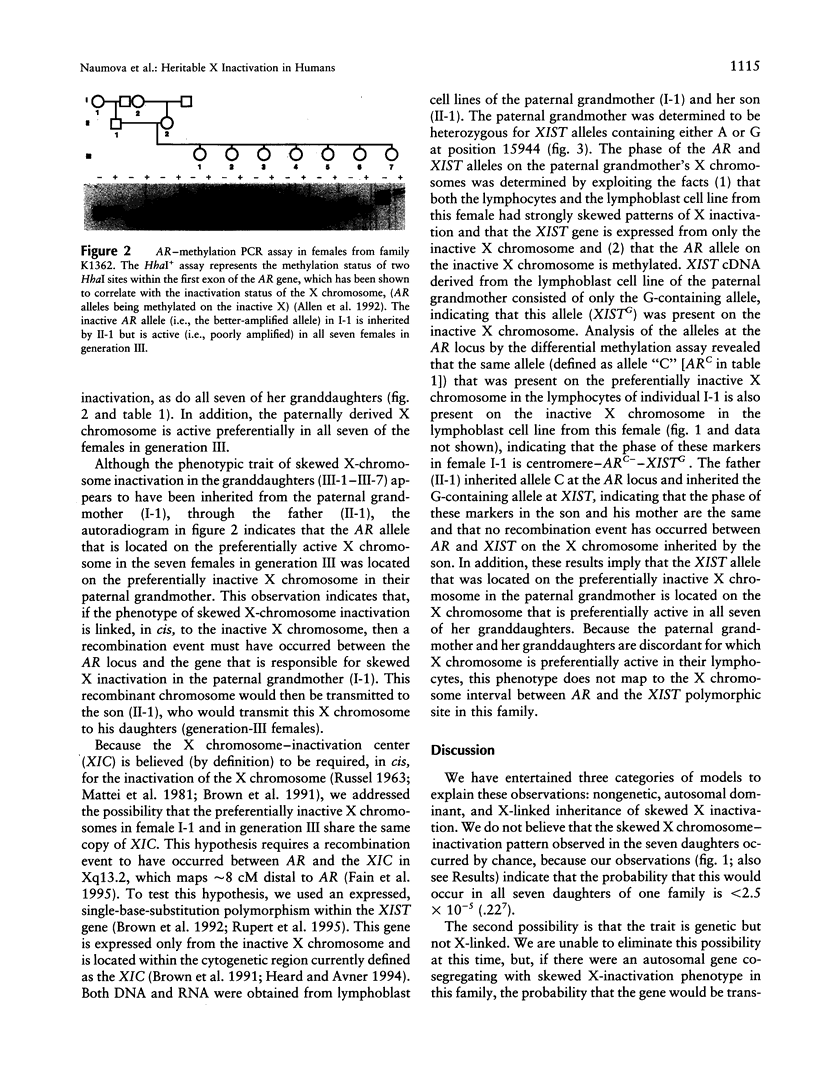
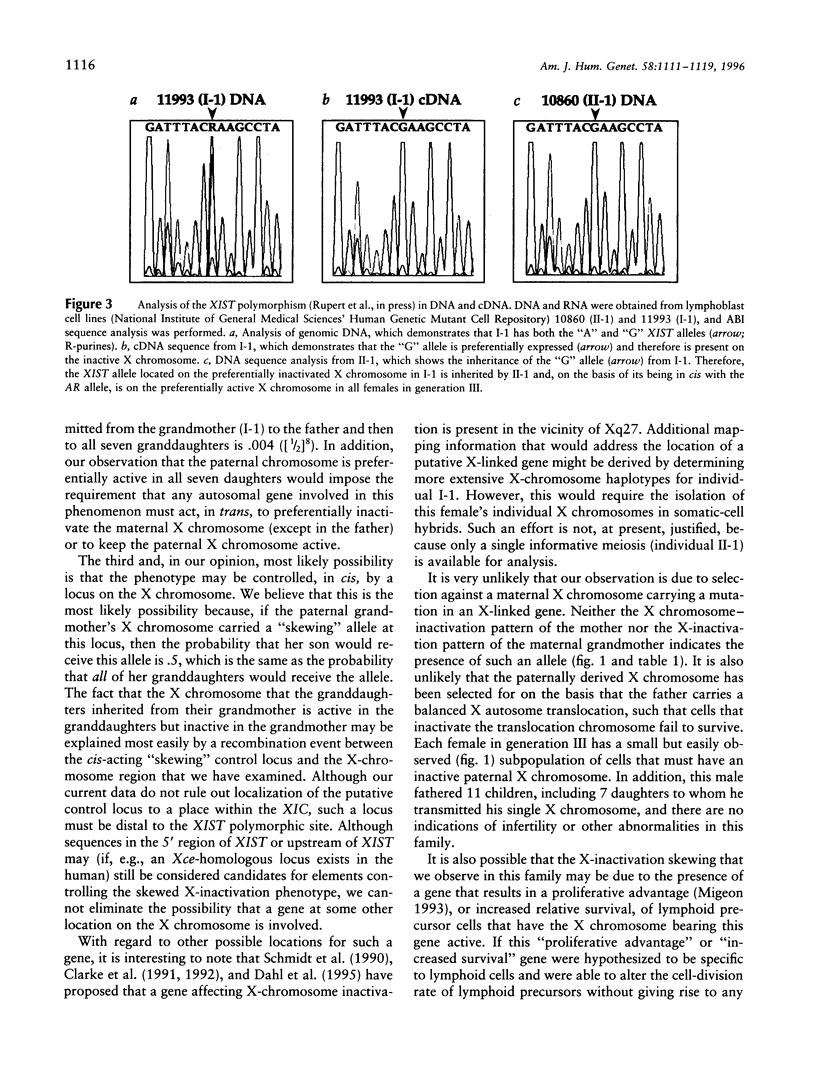
One of the two X chromosomes in each somatic cell of normal human females becomes inactivated very early in embryonic development. Although the inactivation of an X chromosome in any particular somatic cell of the embryonic lineage is thought to be a stochastic and epigenetic event, a strong genetic influence on this process has been described in the mouse. We have attempted to uncover evidence for genetic control of X-chromosome inactivation in the human by examining X chromosome-inactivation patterns in 255 females from 36 three-generation pedigrees, to determine whether this quantitative character exhibits evidence of heritability. We have found one family in which all seven daughters of one male and the mother of this male have highly skewed patterns of X-chromosome inactivation, suggesting strongly that this quantitative character is controlled by one or more X-linked genes in some families.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Nachtman R. G., Rosenblatt H. M., Belmont J. W. Application of carrier testing to genetic counseling for X-linked agammaglobulinemia. Am J Hum Genet. 1994 Jan;54(1):25–35. [PMC free article] [PubMed] [Google Scholar]
- Allen R. C., Zoghbi H. Y., Moseley A. B., Rosenblatt H. M., Belmont J. W. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992 Dec;51(6):1229–1239. [PMC free article] [PubMed] [Google Scholar]
- Ariel M., Robinson E., McCarrey J. R., Cedar H. Gamete-specific methylation correlates with imprinting of the murine Xist gene. Nat Genet. 1995 Mar;9(3):312–315. doi: 10.1038/ng0395-312. [DOI] [PubMed] [Google Scholar]
- Borsani G., Tonlorenzi R., Simmler M. C., Dandolo L., Arnaud D., Capra V., Grompe M., Pizzuti A., Muzny D., Lawrence C. Characterization of a murine gene expressed from the inactive X chromosome. Nature. 1991 May 23;351(6324):325–329. doi: 10.1038/351325a0. [DOI] [PubMed] [Google Scholar]
- Brockdorff N., Ashworth A., Kay G. F., Cooper P., Smith S., McCabe V. M., Norris D. P., Penny G. D., Patel D., Rastan S. Conservation of position and exclusive expression of mouse Xist from the inactive X chromosome. Nature. 1991 May 23;351(6324):329–331. doi: 10.1038/351329a0. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Ballabio A., Rupert J. L., Lafreniere R. G., Grompe M., Tonlorenzi R., Willard H. F. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991 Jan 3;349(6304):38–44. doi: 10.1038/349038a0. [DOI] [PubMed] [Google Scholar]
- Brown C. J., Flenniken A. M., Williams B. R., Willard H. F. X chromosome inactivation of the human TIMP gene. Nucleic Acids Res. 1990 Jul 25;18(14):4191–4195. doi: 10.1093/nar/18.14.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. J., Hendrich B. D., Rupert J. L., Lafrenière R. G., Xing Y., Lawrence J., Willard H. F. The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell. 1992 Oct 30;71(3):527–542. doi: 10.1016/0092-8674(92)90520-m. [DOI] [PubMed] [Google Scholar]
- Brown R. M., Brown G. K. X chromosome inactivation and the diagnosis of X linked disease in females. J Med Genet. 1993 Mar;30(3):177–184. doi: 10.1136/jmg.30.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattanach B. M., Williams C. E. Evidence of non-random X chromosome activity in the mouse. Genet Res. 1972 Jun;19(3):229–240. doi: 10.1017/s001667230001449x. [DOI] [PubMed] [Google Scholar]
- Clarke J. T., Greer W. L., Strasberg P. M., Pearce R. D., Skomorowski M. A., Ray P. N. Hunter disease (mucopolysaccharidosis type II) associated with unbalanced inactivation of the X chromosomes in a karyotypically normal girl. Am J Hum Genet. 1991 Aug;49(2):289–297. [PMC free article] [PubMed] [Google Scholar]
- Clarke J. T., Wilson P. J., Morris C. P., Hopwood J. J., Richards R. I., Sutherland G. R., Ray P. N. Characterization of a deletion at Xq27-q28 associated with unbalanced inactivation of the nonmutant X chromosome. Am J Hum Genet. 1992 Aug;51(2):316–322. [PMC free article] [PubMed] [Google Scholar]
- Dahl N., Hu L. J., Chery M., Fardeau M., Gilgenkrantz S., Nivelon-Chevallier A., Sidaner-Noisette I., Mugneret F., Gouyon J. B., Gal A. Myotubular myopathy in a girl with a deletion at Xq27-q28 and unbalanced X inactivation assigns the MTM1 gene to a 600-kb region. Am J Hum Genet. 1995 May;56(5):1108–1115. [PMC free article] [PubMed] [Google Scholar]
- Edwards A., Hammond H. A., Jin L., Caskey C. T., Chakraborty R. Genetic variation at five trimeric and tetrameric tandem repeat loci in four human population groups. Genomics. 1992 Feb;12(2):241–253. doi: 10.1016/0888-7543(92)90371-x. [DOI] [PubMed] [Google Scholar]
- Fain P. R., Kort E. N., Chance P. F., Nguyen K., Redd D. F., Econs M. J., Barker D. F. A 2D crossover-based map of the human X chromosome as a model for map integration. Nat Genet. 1995 Mar;9(3):261–266. doi: 10.1038/ng0395-261. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Suthers G. K., Wilkie A. O., Buckle V. J., Higgs D. R. X-linked alpha-thalassemia/mental retardation (ATR-X) syndrome: localization to Xq12-q21.31 by X inactivation and linkage analysis. Am J Hum Genet. 1992 Nov;51(5):1136–1149. [PMC free article] [PubMed] [Google Scholar]
- Harrison K. B., Warburton D. Preferential X-chromosome activity in human female placental tissues. Cytogenet Cell Genet. 1986;41(3):163–168. doi: 10.1159/000132221. [DOI] [PubMed] [Google Scholar]
- Harrison K. B. X-chromosome inactivation in the human cytotrophoblast. Cytogenet Cell Genet. 1989;52(1-2):37–41. doi: 10.1159/000132835. [DOI] [PubMed] [Google Scholar]
- Heard E., Avner P. Role play in X-inactivation. Hum Mol Genet. 1994;3(Spec No):1481–1485. doi: 10.1093/hmg/3.suppl_1.1481. [DOI] [PubMed] [Google Scholar]
- Johnston P. G., Cattanach B. M. Controlling elements in the mouse. IV. Evidence of non-random X-inactivation. Genet Res. 1981 Apr;37(2):151–160. doi: 10.1017/s0016672300020127. [DOI] [PubMed] [Google Scholar]
- Kay G. F., Penny G. D., Patel D., Ashworth A., Brockdorff N., Rastan S. Expression of Xist during mouse development suggests a role in the initiation of X chromosome inactivation. Cell. 1993 Jan 29;72(2):171–182. doi: 10.1016/0092-8674(93)90658-d. [DOI] [PubMed] [Google Scholar]
- La Spada A. R., Wilson E. M., Lubahn D. B., Harding A. E., Fischbeck K. H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991 Jul 4;352(6330):77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- Leisti J. T., Kaback M. M., Rimoin D. L. Human X-autosome translocations: differential inactivation of the X chromosome in a kindred with an X-9 translocation. Am J Hum Genet. 1975 Jul;27(4):441–453. [PMC free article] [PubMed] [Google Scholar]
- Lyon M. F. The William Allan memorial award address: X-chromosome inactivation and the location and expression of X-linked genes. Am J Hum Genet. 1988 Jan;42(1):8–16. [PMC free article] [PubMed] [Google Scholar]
- Mattei M. G., Mattei J. F., Vidal I., Giraud F. Structural anomalies of the X chromosome and inactivation center. Hum Genet. 1981;56(3):401–408. doi: 10.1007/BF00274702. [DOI] [PubMed] [Google Scholar]
- Migeon B. R. The postulated X-inactivation center at Xq27 is most reasonably explained by ascertainment bias: heterozygous expression of recessive mutations is a powerful means of detecting unbalanced X inactivation. Am J Hum Genet. 1993 Feb;52(2):431–434. [PMC free article] [PubMed] [Google Scholar]
- Migeon B. R. The postulated X-inactivation center at Xq27 is most reasonably explained by ascertainment bias: heterozygous expression of recessive mutations is a powerful means of detecting unbalanced X inactivation. Am J Hum Genet. 1993 Feb;52(2):431–434. [PMC free article] [PubMed] [Google Scholar]
- NANCE W. E. GENETIC TESTS WITH A SEX-LINKED MARKER: GLUCOSE-6-PHOSPHATE DEHYDROGENASE. Cold Spring Harb Symp Quant Biol. 1964;29:415–425. doi: 10.1101/sqb.1964.029.01.043. [DOI] [PubMed] [Google Scholar]
- Naumova A. K., Olien L., Bird L. M., Slamka C., Fonseca M., Verner A. E., Wang M., Leppert M., Morgan K., Sapienza C. Transmission-ratio distortion of X chromosomes among male offspring of females with skewed X-inactivation. Dev Genet. 1995;17(3):198–205. doi: 10.1002/dvg.1020170304. [DOI] [PubMed] [Google Scholar]
- Orstavik R. E., Tommerup N., Eiklid K., Orstavik K. H. Non-random X chromosome inactivation in an affected twin in a monozygotic twin pair discordant for Wiedemann-Beckwith syndrome. Am J Med Genet. 1995 Mar 27;56(2):210–214. doi: 10.1002/ajmg.1320560219. [DOI] [PubMed] [Google Scholar]
- Pegoraro E., Schimke R. N., Arahata K., Hayashi Y., Stern H., Marks H., Glasberg M. R., Carroll J. E., Taber J. W., Wessel H. B. Detection of new paternal dystrophin gene mutations in isolated cases of dystrophinopathy in females. Am J Hum Genet. 1994 Jun;54(6):989–1003. [PMC free article] [PubMed] [Google Scholar]
- Penny G. D., Kay G. F., Sheardown S. A., Rastan S., Brockdorff N. Requirement for Xist in X chromosome inactivation. Nature. 1996 Jan 11;379(6561):131–137. doi: 10.1038/379131a0. [DOI] [PubMed] [Google Scholar]
- Puck J. M., Stewart C. C., Nussbaum R. L. Maximum-likelihood analysis of human T-cell X chromosome inactivation patterns: normal women versus carriers of X-linked severe combined immunodeficiency. Am J Hum Genet. 1992 Apr;50(4):742–748. [PMC free article] [PubMed] [Google Scholar]
- RUSSELL L. B. Mammalian X-chromosome action: inactivation limited in spread and region of origin. Science. 1963 May 31;140(3570):976–978. doi: 10.1126/science.140.3570.976. [DOI] [PubMed] [Google Scholar]
- Ropers H. H., Wolff G., Hitzeroth H. W. Preferential X inactivation in human placenta membranes: is the paternal X inactive in early embryonic development of female mammals? Hum Genet. 1978 Sep 19;43(3):265–273. doi: 10.1007/BF00278833. [DOI] [PubMed] [Google Scholar]
- Rupert J. L., Brown C. J., Willard H. F. Direct detection of non-random X chromosome inactivation by use of a transcribed polymorphism in the XIST gene. Eur J Hum Genet. 1995;3(6):333–343. doi: 10.1159/000472322. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Certoma A., Du Sart D., Kalitsis P., Leversha M., Fowler K., Sheffield L., Jack I., Danks D. M. Unusual X chromosome inactivation in a mentally retarded girl with an interstitial deletion Xq27: implications for the fragile X syndrome. Hum Genet. 1990 Mar;84(4):347–352. doi: 10.1007/BF00196232. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Du Sart D. Functional disomies of the X chromosome influence the cell selection and hence the X inactivation pattern in females with balanced X-autosome translocations: a review of 122 cases. Am J Med Genet. 1992 Jan 15;42(2):161–169. doi: 10.1002/ajmg.1320420205. [DOI] [PubMed] [Google Scholar]
- Simmler M. C., Cattanach B. M., Rasberry C., Rougeulle C., Avner P. Mapping the murine Xce locus with (CA)n repeats. Mamm Genome. 1993 Sep;4(9):523–530. doi: 10.1007/BF00364788. [DOI] [PubMed] [Google Scholar]
- Takagi N., Sasaki M. Preferential inactivation of the paternally derived X chromosome in the extraembryonic membranes of the mouse. Nature. 1975 Aug 21;256(5519):640–642. doi: 10.1038/256640a0. [DOI] [PubMed] [Google Scholar]
- Wengler G., Gorlin J. B., Williamson J. M., Rosen F. S., Bing D. H. Nonrandom inactivation of the X chromosome in early lineage hematopoietic cells in carriers of Wiskott-Aldrich syndrome. Blood. 1995 May 1;85(9):2471–2477. [PubMed] [Google Scholar]
- West J. D., Chapman V. M. Variation for X chromosome expression in mice detected by electrophoresis of phosphoglycerate kinase. Genet Res. 1978 Aug;32(1):91–102. doi: 10.1017/s0016672300018565. [DOI] [PubMed] [Google Scholar]
- West J. D., Frels W. I., Chapman V. M., Papaioannou V. E. Preferential expression of the maternally derived X chromosome in the mouse yolk sac. Cell. 1977 Dec;12(4):873–882. doi: 10.1016/0092-8674(77)90151-9. [DOI] [PubMed] [Google Scholar]
- Zabel B. U., Baumann W. A., Pirntke W., Gerhard-Ratschow K. X-inactivation pattern in three cases of X/autosome translocation. Am J Med Genet. 1978;1(3):309–317. doi: 10.1002/ajmg.1320010307. [DOI] [PubMed] [Google Scholar]
- Zuccotti M., Monk M. Methylation of the mouse Xist gene in sperm and eggs correlates with imprinted Xist expression and paternal X-inactivation. Nat Genet. 1995 Mar;9(3):316–320. doi: 10.1038/ng0395-316. [DOI] [PubMed] [Google Scholar]



