Abstract
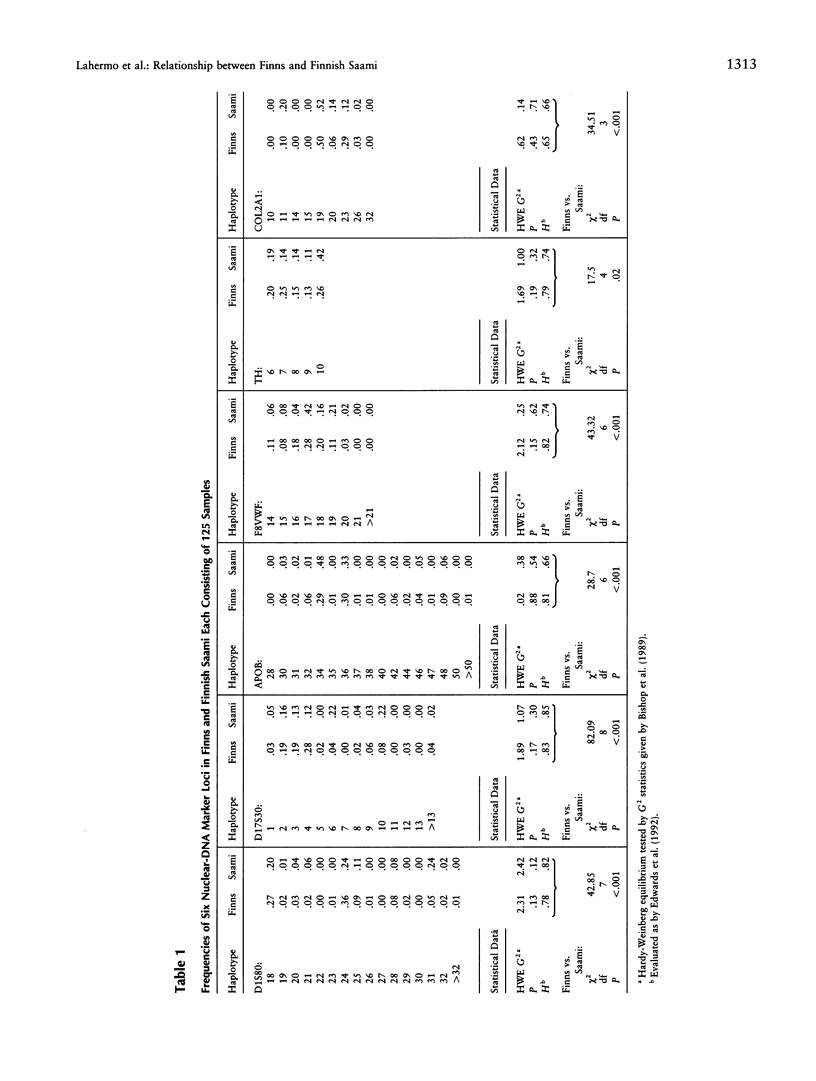
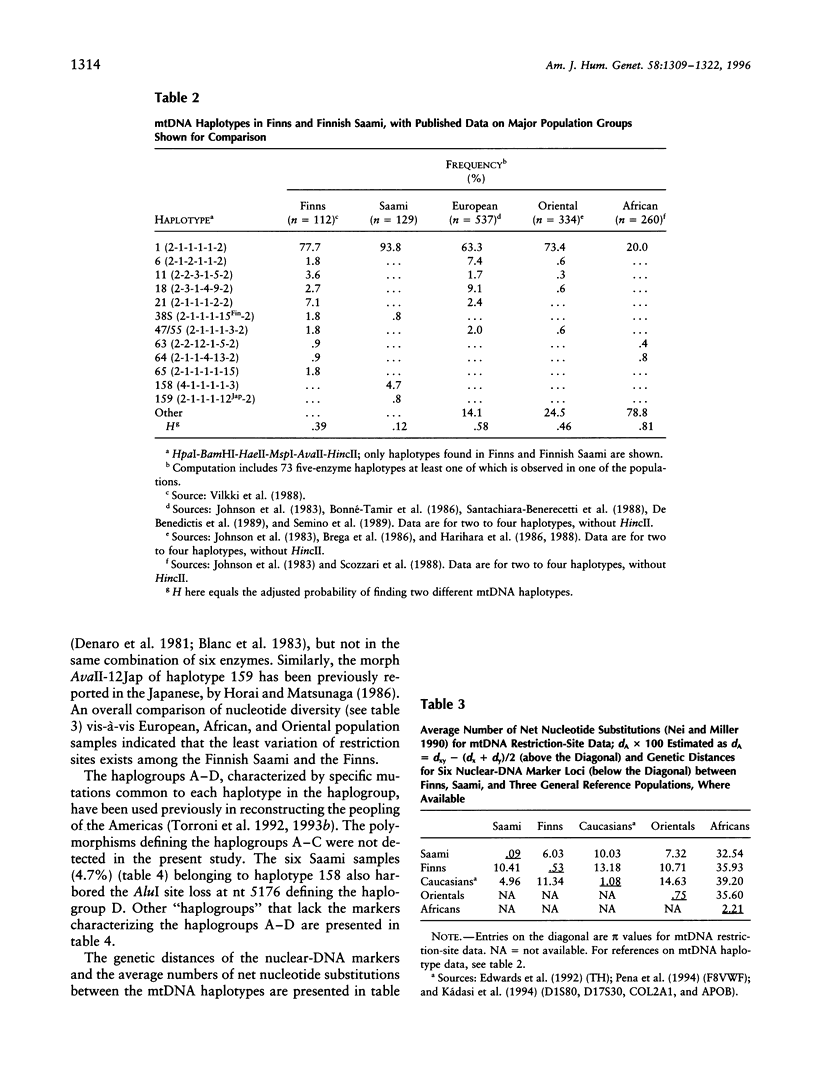
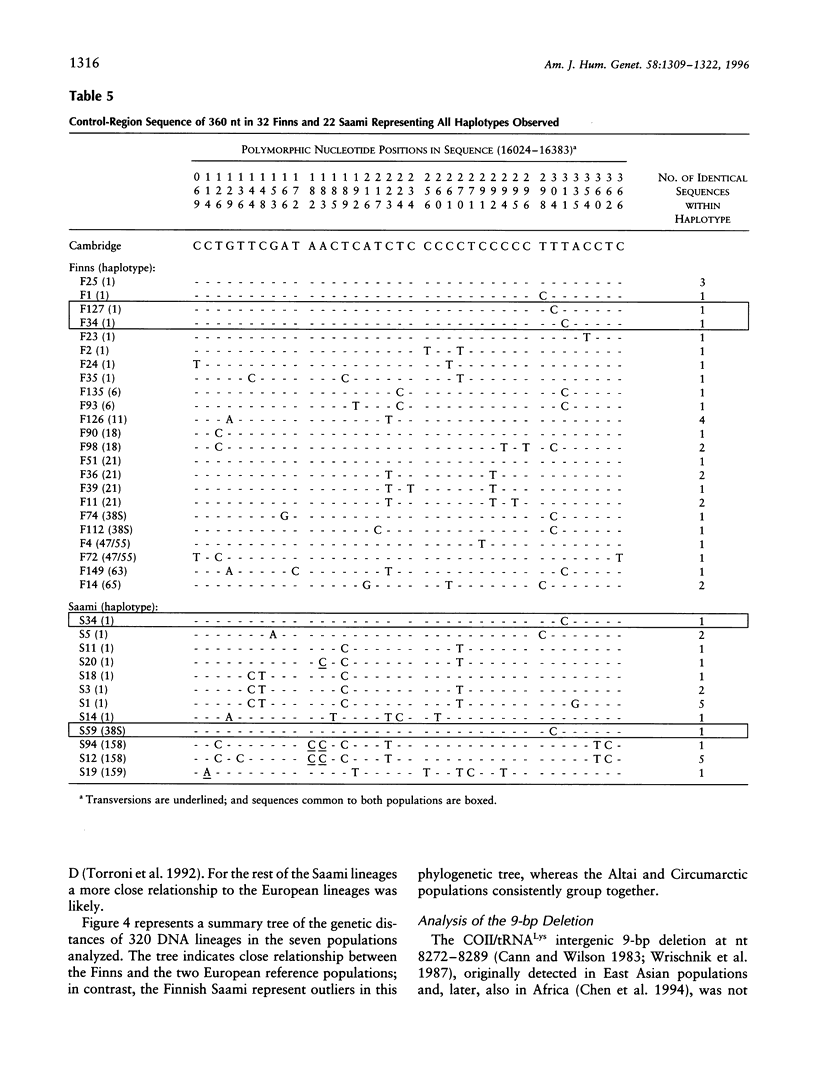
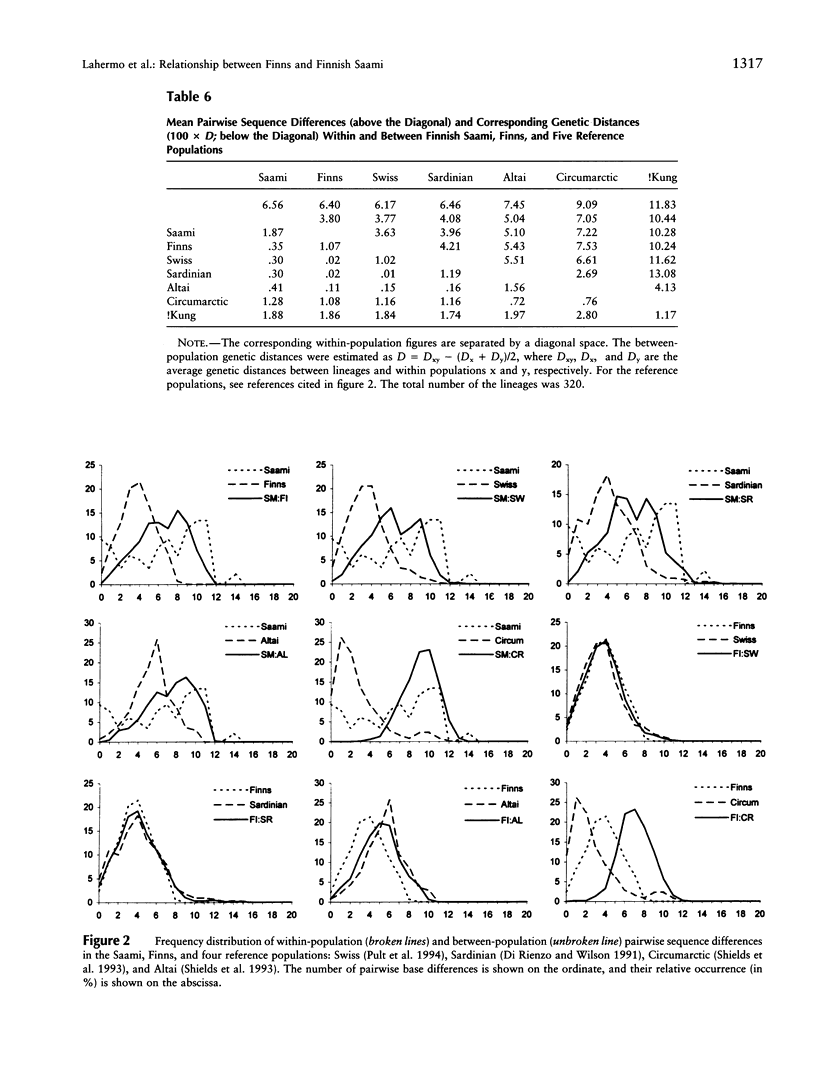
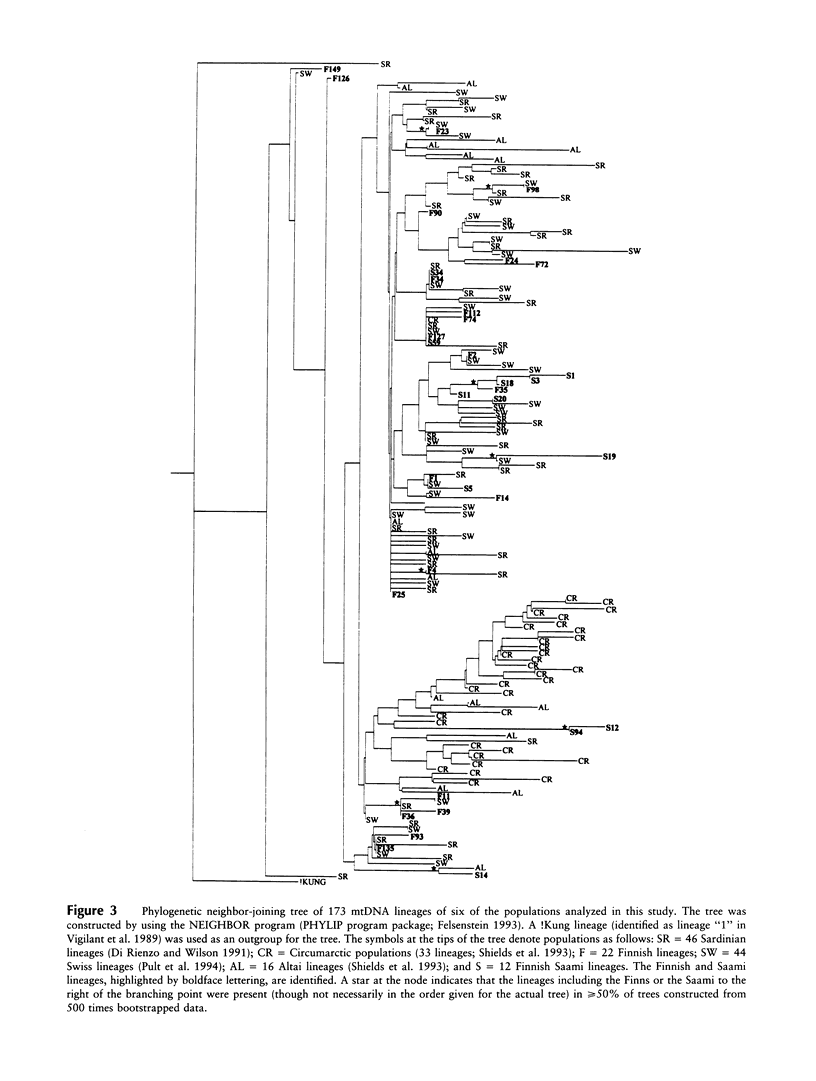
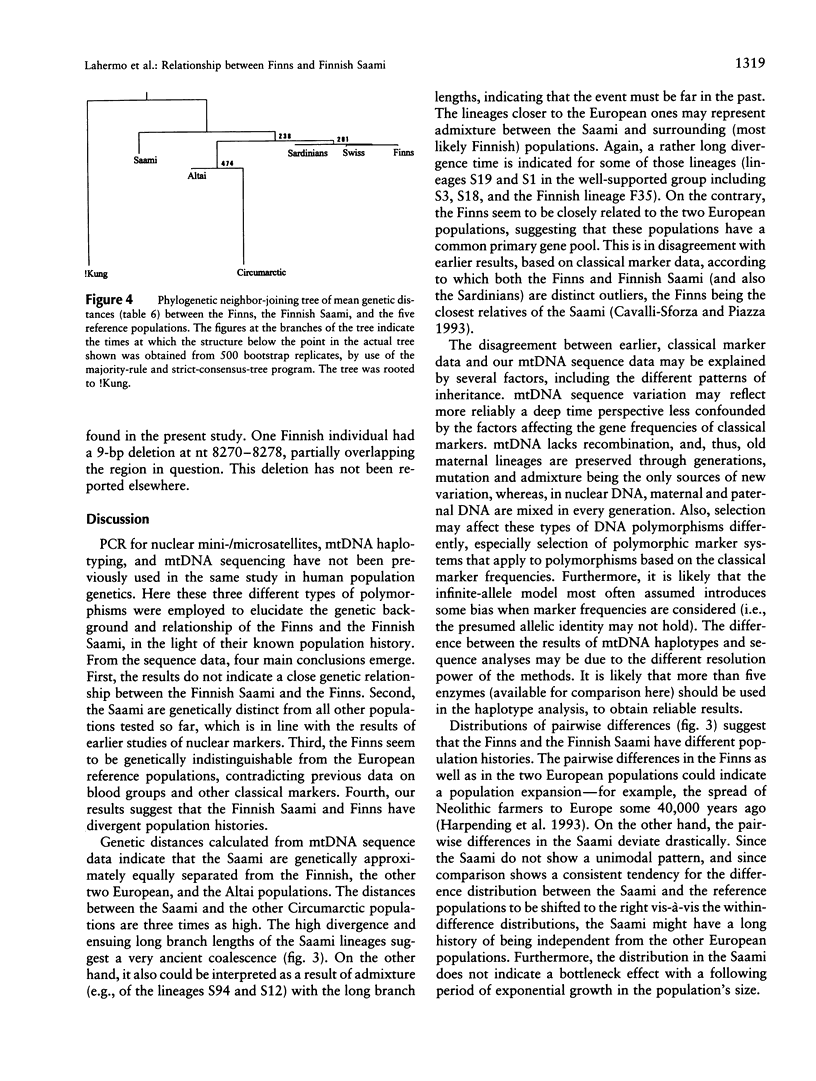
The genetic relationships between two Finno-Ugric-speaking populations, the Finns and the Finnish Saami (Lapps), were studied by using PCR for six nuclear-DNA marker loci, mitochondrial restriction-site polymorphism, and sequence variation of a 360-bp segment of the mitochondrial control region. The allele frequencies of each of the nuclear-DNA marker loci and the frequencies of mtDNA restriction haplotypes were significantly different between the populations. The Saami showed exceptionally low variation in their mtDNA restriction sites. The 9-bp deletion common in East Asian populations was not observed, nor did the haplotype data fit into the haplogroup categorization of Torroni et al. The average number of nucleotide substitutions from the mtDNA haplotype data indicated that the Finnish Saami may be closer to the Finns than to the other reference populations, whereas nuclear DNA suggested that the Finns are more closely related to the European reference populations than to the Finnish Saami. The similarity of the Finns to the other Europeans was even more pronounced according to the sequence data. We were unable to distinguish between the Finns and either the Swiss or Sardinian reference populations, whereas the Finnish Saami clearly stood apart. The Finnish Saami are distinct from other Circumarctic populations, although two of the lineages found among the Saami showed closer relationship to the Circumarctic than to the European lineages. The sequence data indicated an exceptionally high divergence for the Saami mtDNA control lineages. The distribution of the pairwise nucleotide differences in the Saami suggested that this population has not experienced an expansion similar to what was indicated for the Finns and the reference populations.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Graves G., Budowle B. Polymerase chain reaction amplification products separated on rehydratable polyacrylamide gels and stained with silver. Biotechniques. 1989 Jul-Aug;7(7):736–744. [PubMed] [Google Scholar]
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Blanc H., Chen K. H., D'Amore M. A., Wallace D. C. Amino acid change associated with the major polymorphic Hinc II site of Oriental and Caucasian mitochondrial DNAs. Am J Hum Genet. 1983 Mar;35(2):167–176. [PMC free article] [PubMed] [Google Scholar]
- Boerwinkle E., Xiong W. J., Fourest E., Chan L. Rapid typing of tandemly repeated hypervariable loci by the polymerase chain reaction: application to the apolipoprotein B 3' hypervariable region. Proc Natl Acad Sci U S A. 1989 Jan;86(1):212–216. doi: 10.1073/pnas.86.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonné-Tamir B., Johnson M. J., Natali A., Wallace D. C., Cavalli-Sforza L. L. Human mitochondrial DNA types in two Israeli populations--a comparative study at the DNA level. Am J Hum Genet. 1986 Mar;38(3):341–351. [PMC free article] [PubMed] [Google Scholar]
- Brega A., Gardella R., Semino O., Morpurgo G., Astaldi Ricotti G. B., Wallace D. C., Santachiara Benerecetti A. S. Genetic studies on the Tharu population of Nepal: restriction endonuclease polymorphisms of mitochondrial DNA. Am J Hum Genet. 1986 Oct;39(4):502–512. [PMC free article] [PubMed] [Google Scholar]
- Brown W. M., George M., Jr, Wilson A. C. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann R. L., Brown W. M., Wilson A. C. Polymorphic sites and the mechanism of evolution in human mitochondrial DNA. Genetics. 1984 Mar;106(3):479–499. doi: 10.1093/genetics/106.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann R. L., Wilson A. C. Length mutations in human mitochondrial DNA. Genetics. 1983 Aug;104(4):699–711. doi: 10.1093/genetics/104.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli-Sforza L. L., Piazza A. Human genomic diversity in Europe: a summary of recent research and prospects for the future. Eur J Hum Genet. 1993;1(1):3–18. doi: 10.1159/000472383. [DOI] [PubMed] [Google Scholar]
- Chou Q., Russell M., Birch D. E., Raymond J., Bloch W. Prevention of pre-PCR mis-priming and primer dimerization improves low-copy-number amplifications. Nucleic Acids Res. 1992 Apr 11;20(7):1717–1723. doi: 10.1093/nar/20.7.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deka R., Chakroborty R., Ferrell R. E. A population genetic study of six VNTR loci in three ethnically defined populations. Genomics. 1991 Sep;11(1):83–92. doi: 10.1016/0888-7543(91)90104-m. [DOI] [PubMed] [Google Scholar]
- Denaro M., Blanc H., Johnson M. J., Chen K. H., Wilmsen E., Cavalli-Sforza L. L., Wallace D. C. Ethnic variation in Hpa 1 endonuclease cleavage patterns of human mitochondrial DNA. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5768–5772. doi: 10.1073/pnas.78.9.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rienzo A., Wilson A. C. Branching pattern in the evolutionary tree for human mitochondrial DNA. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1597–1601. doi: 10.1073/pnas.88.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A., Civitello A., Hammond H. A., Caskey C. T. DNA typing and genetic mapping with trimeric and tetrameric tandem repeats. Am J Hum Genet. 1991 Oct;49(4):746–756. [PMC free article] [PubMed] [Google Scholar]
- Edwards A., Hammond H. A., Jin L., Caskey C. T., Chakraborty R. Genetic variation at five trimeric and tetrameric tandem repeat loci in four human population groups. Genomics. 1992 Feb;12(2):241–253. doi: 10.1016/0888-7543(92)90371-x. [DOI] [PubMed] [Google Scholar]
- Eriksson A. W. Genetic polymorphisms in Finno-Ugrian populations. Finns, Lapps and Maris. Isr J Med Sci. 1973 Sep-Oct;9(9):1156–1170. [PubMed] [Google Scholar]
- Giles R. E., Blanc H., Cann H. M., Wallace D. C. Maternal inheritance of human mitochondrial DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6715–6719. doi: 10.1073/pnas.77.11.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmino C. R., Piazza A., Menozzi P., Cavalli-Sforza L. L. Uralic genes in Europe. Am J Phys Anthropol. 1990 Sep;83(1):57–68. doi: 10.1002/ajpa.1330830107. [DOI] [PubMed] [Google Scholar]
- Harihara S., Hirai M., Omoto K. Mitochondrial DNA polymorphism in Japanese living in Hokkaido. Jinrui Idengaku Zasshi. 1986 Jun;31(2):73–83. doi: 10.1007/BF01871401. [DOI] [PubMed] [Google Scholar]
- Harihara S., Saitou N., Hirai M., Gojobori T., Park K. S., Misawa S., Ellepola S. B., Ishida T., Omoto K. Mitochondrial DNA polymorphism among five Asian populations. Am J Hum Genet. 1988 Aug;43(2):134–143. [PMC free article] [PubMed] [Google Scholar]
- Horai S., Matsunaga E. Mitochondrial DNA polymorphism in Japanese. II. Analysis with restriction enzymes of four or five base pair recognition. Hum Genet. 1986 Feb;72(2):105–117. doi: 10.1007/BF00283927. [DOI] [PubMed] [Google Scholar]
- Horn G. T., Richards B., Klinger K. W. Amplification of a highly polymorphic VNTR segment by the polymerase chain reaction. Nucleic Acids Res. 1989 Mar 11;17(5):2140–2140. doi: 10.1093/nar/17.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Thein S. L. Hypervariable 'minisatellite' regions in human DNA. Nature. 1985 Mar 7;314(6006):67–73. doi: 10.1038/314067a0. [DOI] [PubMed] [Google Scholar]
- Johnson M. J., Wallace D. C., Ferris S. D., Rattazzi M. C., Cavalli-Sforza L. L. Radiation of human mitochondria DNA types analyzed by restriction endonuclease cleavage patterns. J Mol Evol. 1983;19(3-4):255–271. doi: 10.1007/BF02099973. [DOI] [PubMed] [Google Scholar]
- Kasai K., Nakamura Y., White R. Amplification of a variable number of tandem repeats (VNTR) locus (pMCT118) by the polymerase chain reaction (PCR) and its application to forensic science. J Forensic Sci. 1990 Sep;35(5):1196–1200. [PubMed] [Google Scholar]
- Kimpton C., Walton A., Gill P. A further tetranucleotide repeat polymorphism in the vWF gene. Hum Mol Genet. 1992 Jul;1(4):287–287. doi: 10.1093/hmg/1.4.287. [DOI] [PubMed] [Google Scholar]
- Kádasi L., Gécz J., Feráková I., Lubyová B., Bohusová T., Feráková E., Poláková H. Distribution of ApoBII, MCT118 (D1S80), YNZ22 (D17S30), and COL2A1 Amp-FLPs (amplified fragment length polymorphisms) in Caucasoid population of Slovakia. Gene Geogr. 1994 Aug;8(2):121–127. [PubMed] [Google Scholar]
- Litt M., Luty J. A. A hypervariable microsatellite revealed by in vitro amplification of a dinucleotide repeat within the cardiac muscle actin gene. Am J Hum Genet. 1989 Mar;44(3):397–401. [PMC free article] [PubMed] [Google Scholar]
- Nei M., Miller J. C. A simple method for estimating average number of nucleotide substitutions within and between populations from restriction data. Genetics. 1990 Aug;125(4):873–879. doi: 10.1093/genetics/125.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M. The theory of genetic distance and evolution of human races. Jinrui Idengaku Zasshi. 1978 Dec;23(4):341–369. doi: 10.1007/BF01908190. [DOI] [PubMed] [Google Scholar]
- Nevanlinna H. R. The Finnish population structure. A genetic and genealogical study. Hereditas. 1972;71(2):195–236. doi: 10.1111/j.1601-5223.1972.tb01021.x. [DOI] [PubMed] [Google Scholar]
- Pena S. D., de Souza K. T., de Andrade M., Chakraborty R. Allelic associations of two polymorphic microsatellites in intron 40 of the human von Willebrand factor gene. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):723–727. doi: 10.1073/pnas.91.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pult I., Sajantila A., Simanainen J., Georgiev O., Schaffner W., Päbo S. Mitochondrial DNA sequences from Switzerland reveal striking homogeneity of European populations. Biol Chem Hoppe Seyler. 1994 Dec;375(12):837–840. [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sajantila A., Budowle B., Ström M., Johnsson V., Lukka M., Peltonen L., Ehnholm C. PCR amplification of alleles at the DIS80 locus: comparison of a Finnish and a North American Caucasian population sample, and forensic casework evaluation. Am J Hum Genet. 1992 Apr;50(4):816–825. [PMC free article] [PubMed] [Google Scholar]
- Sajantila A., Lahermo P., Anttinen T., Lukka M., Sistonen P., Savontaus M. L., Aula P., Beckman L., Tranebjaerg L., Gedde-Dahl T. Genes and languages in Europe: an analysis of mitochondrial lineages. Genome Res. 1995 Aug;5(1):42–52. doi: 10.1101/gr.5.1.42. [DOI] [PubMed] [Google Scholar]
- Sajantila A., Lukka M. Improved separation of PCR amplified VNTR alleles by a vertical polyacrylamide gel electrophoresis. Int J Legal Med. 1993;105(6):355–359. doi: 10.1007/BF01222121. [DOI] [PubMed] [Google Scholar]
- Sajantila A., Puomilahti S., Johnsson V., Ehnholm C. Amplification of reproducible allele markers for amplified fragment length polymorphism analysis. Biotechniques. 1992 Jan;12(1):16, 18, 20-2. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santachiara Benerecetti A. S., Scozzari R., Semino O., Torroni A., Brega A., Wallace D. C. Mitochondrial DNA polymorphisms in Italy. II. Molecular analysis of new and rare morphs from Sardinia and Rome. Ann Hum Genet. 1988 Jan;52(Pt 1):39–56. doi: 10.1111/j.1469-1809.1988.tb01076.x. [DOI] [PubMed] [Google Scholar]
- Sartoris S., Varetto O., Migone N., Cappello N., Piazza A., Ferrara G. B., Ceppellini R. Mitochondrial DNA polymorphism in four Sardinian villages. Ann Hum Genet. 1988 Oct;52(Pt 4):327–340. doi: 10.1111/j.1469-1809.1988.tb01112.x. [DOI] [PubMed] [Google Scholar]
- Scozzari R., Torroni A., Semino O., Sirugo G., Brega A., Santachiara-Benerecetti A. S. Genetic studies on the Senegal population. I. Mitochondrial DNA polymorphisms. Am J Hum Genet. 1988 Oct;43(4):534–544. [PMC free article] [PubMed] [Google Scholar]
- Semino O., Torroni A., Scozzari R., Brega A., De Benedictis G., Santachiara Benerecetti A. S. Mitochondrial DNA polymorphisms in Italy. III. Population data from Sicily: a possible quantitation of maternal African ancestry. Ann Hum Genet. 1989 May;53(Pt 2):193–202. doi: 10.1111/j.1469-1809.1989.tb01784.x. [DOI] [PubMed] [Google Scholar]
- Shields G. F., Schmiechen A. M., Frazier B. L., Redd A., Voevoda M. I., Reed J. K., Ward R. H. mtDNA sequences suggest a recent evolutionary divergence for Beringian and northern North American populations. Am J Hum Genet. 1993 Sep;53(3):549–562. [PMC free article] [PubMed] [Google Scholar]
- Stoneking M., Hedgecock D., Higuchi R. G., Vigilant L., Erlich H. A. Population variation of human mtDNA control region sequences detected by enzymatic amplification and sequence-specific oligonucleotide probes. Am J Hum Genet. 1991 Feb;48(2):370–382. [PMC free article] [PubMed] [Google Scholar]
- Torroni A., Lott M. T., Cabell M. F., Chen Y. S., Lavergne L., Wallace D. C. mtDNA and the origin of Caucasians: identification of ancient Caucasian-specific haplogroups, one of which is prone to a recurrent somatic duplication in the D-loop region. Am J Hum Genet. 1994 Oct;55(4):760–776. [PMC free article] [PubMed] [Google Scholar]
- Torroni A., Schurr T. G., Cabell M. F., Brown M. D., Neel J. V., Larsen M., Smith D. G., Vullo C. M., Wallace D. C. Asian affinities and continental radiation of the four founding Native American mtDNAs. Am J Hum Genet. 1993 Sep;53(3):563–590. [PMC free article] [PubMed] [Google Scholar]
- Torroni A., Schurr T. G., Yang C. C., Szathmary E. J., Williams R. C., Schanfield M. S., Troup G. A., Knowler W. C., Lawrence D. N., Weiss K. M. Native American mitochondrial DNA analysis indicates that the Amerind and the Nadene populations were founded by two independent migrations. Genetics. 1992 Jan;130(1):153–162. doi: 10.1093/genetics/130.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torroni A., Sukernik R. I., Schurr T. G., Starikorskaya Y. B., Cabell M. F., Crawford M. H., Comuzzie A. G., Wallace D. C. mtDNA variation of aboriginal Siberians reveals distinct genetic affinities with Native Americans. Am J Hum Genet. 1993 Sep;53(3):591–608. [PMC free article] [PubMed] [Google Scholar]
- Vigilant L., Pennington R., Harpending H., Kocher T. D., Wilson A. C. Mitochondrial DNA sequences in single hairs from a southern African population. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9350–9354. doi: 10.1073/pnas.86.23.9350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilkki J., Savontaus M. L., Nikoskelainen E. K. Human mitochondrial DNA types in Finland. Hum Genet. 1988 Dec;80(4):317–321. doi: 10.1007/BF00273643. [DOI] [PubMed] [Google Scholar]
- Walsh P. S., Metzger D. A., Higuchi R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques. 1991 Apr;10(4):506–513. [PubMed] [Google Scholar]
- Ward R. H., Frazier B. L., Dew-Jager K., Päbo S. Extensive mitochondrial diversity within a single Amerindian tribe. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8720–8724. doi: 10.1073/pnas.88.19.8720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]
- Wrischnik L. A., Higuchi R. G., Stoneking M., Erlich H. A., Arnheim N., Wilson A. C. Length mutations in human mitochondrial DNA: direct sequencing of enzymatically amplified DNA. Nucleic Acids Res. 1987 Jan 26;15(2):529–542. doi: 10.1093/nar/15.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Seino S., Bell G. I. Human collagen, type II, alpha 1, (COL2A1) gene: VNTR polymorphism detected by gene amplification. Nucleic Acids Res. 1990 May 25;18(10):3102–3102. doi: 10.1093/nar/18.10.3102-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Benedictis G., Rose G., Passarino G., Quagliariello C. Restriction fragment length polymorphism of human mitochondrial DNA in a sample population from Apulia (southern Italy). Ann Hum Genet. 1989 Oct;53(Pt 4):311–318. doi: 10.1111/j.1469-1809.1989.tb01800.x. [DOI] [PubMed] [Google Scholar]