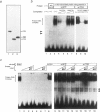
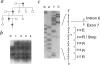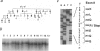Abstract
Waardenburg syndrome type 2 (WS2) is a dominantly inherited disorder characterized by a pigmentation anomaly and hearing impairment due to lack of melanocyte. Previous work has linked a subset of families with WS2 (WS2A) to the MITF gene that encodes a transcription factor with a basic-helix-loop-helix-leucine zipper (bHLH-Zip) motif and that is involved in melanocyte differentiation. Several splice-site and missense mutations have been reported in individuals affected with WS2A. In this report, we have identified two novel point mutations in the MITF gene in affected individuals from two different families with WS2A. The two mutations (C760--> T and C895--> T) create stop codons in exons 7 and 8, respectively. Corresponding mutant alleles predict the truncated proteins lacking HLH-Zip or Zip structure. To understand how these mutations cause WS2 in heterozygotes, we generated mutant MITF cDNAs and used them for DNA-binding and luciferase reporter assays. The mutated MITF proteins lose the DNA-binding activity and fail to transactivate the promoter of tyrosinase, a melanocyte-specific enzyme. However, these mutated proteins do not appear to interfere with the activity of wild-type MITF protein in these assays, indicating that they do not show a dominant-negative effect. These findings suggest that the phenotypes of the two families with WS2A in the present study are caused by loss-of-function mutations in one of the two alleles of the MITF gene, resulting in haploinsufficiency of the MITF protein, the protein necessary for normal development of melanocytes.
Full text
PDF

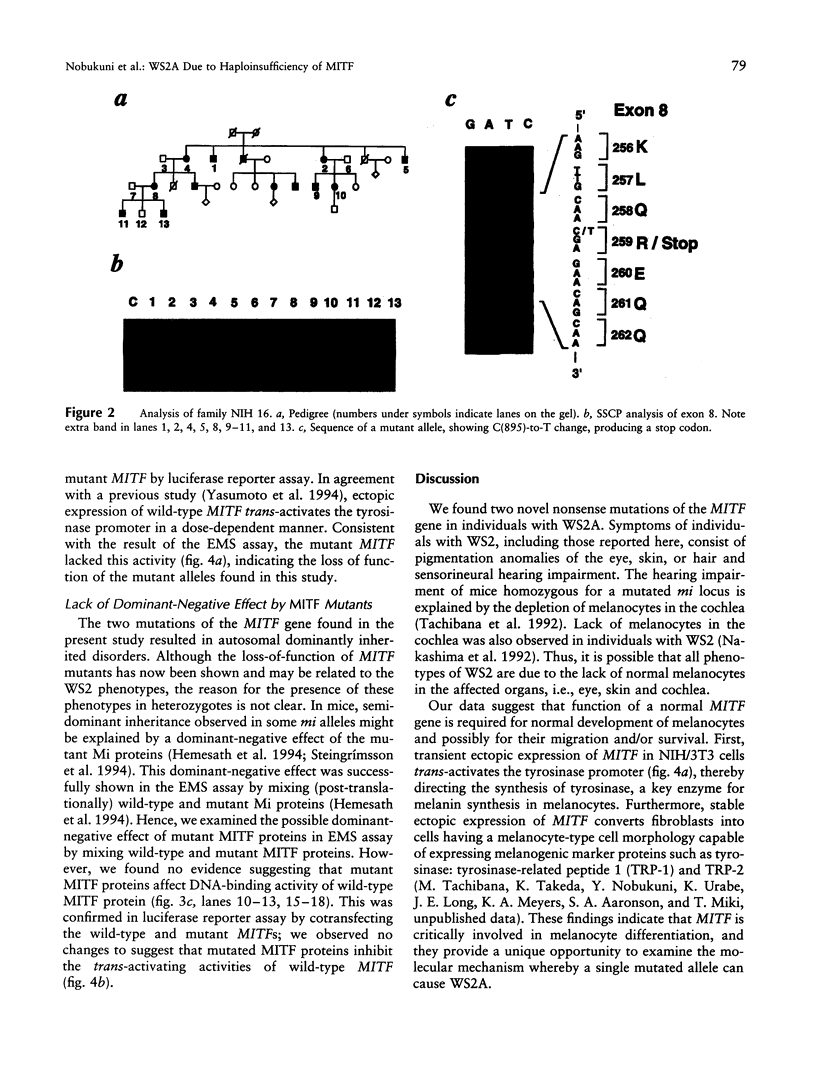
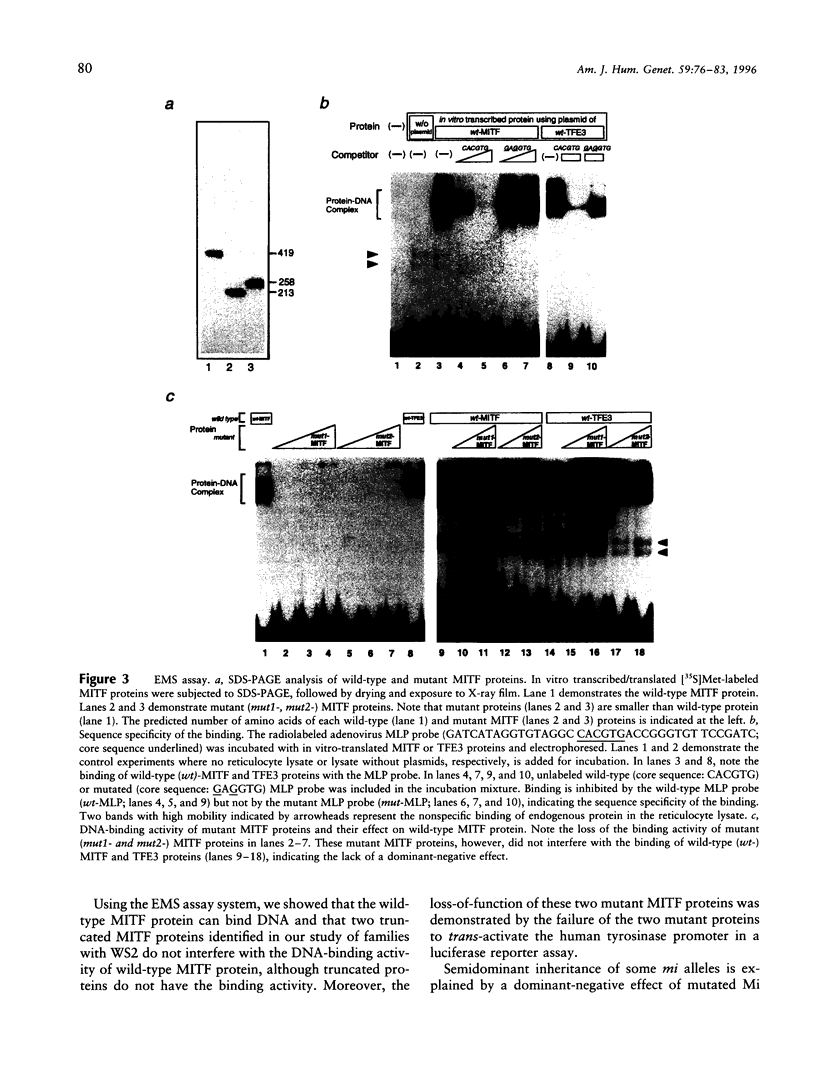
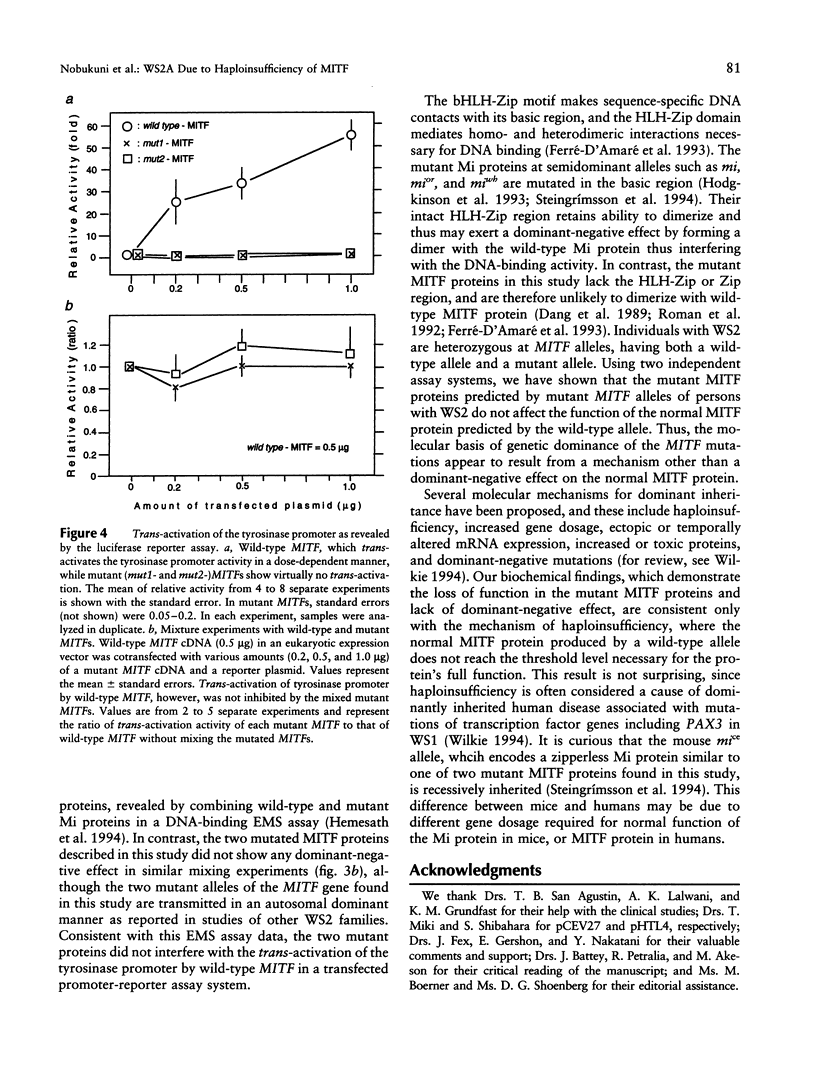


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias S. Genetic heterogeneity in the Waardenburg syndrome. Birth Defects Orig Artic Ser. 1971 Mar;07(4):87–101. [PubMed] [Google Scholar]
- Asher J. H., Jr, Friedman T. B. Mouse and hamster mutants as models for Waardenburg syndromes in humans. J Med Genet. 1990 Oct;27(10):618–626. doi: 10.1136/jmg.27.10.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin C. T., Hoth C. F., Amos J. A., da-Silva E. O., Milunsky A. An exonic mutation in the HuP2 paired domain gene causes Waardenburg's syndrome. Nature. 1992 Feb 13;355(6361):637–638. doi: 10.1038/355637a0. [DOI] [PubMed] [Google Scholar]
- Bentley N. J., Eisen T., Goding C. R. Melanocyte-specific expression of the human tyrosinase promoter: activation by the microphthalmia gene product and role of the initiator. Mol Cell Biol. 1994 Dec;14(12):7996–8006. doi: 10.1128/mcb.14.12.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang C. V., McGuire M., Buckmire M., Lee W. M. Involvement of the 'leucine zipper' region in the oligomerization and transforming activity of human c-myc protein. Nature. 1989 Feb 16;337(6208):664–666. doi: 10.1038/337664a0. [DOI] [PubMed] [Google Scholar]
- Edery P., Attié T., Amiel J., Pelet A., Eng C., Hofstra R. M., Martelli H., Bidaud C., Munnich A., Lyonnet S. Mutation of the endothelin-3 gene in the Waardenburg-Hirschsprung disease (Shah-Waardenburg syndrome). Nat Genet. 1996 Apr;12(4):442–444. doi: 10.1038/ng0496-442. [DOI] [PubMed] [Google Scholar]
- Farrer L. A., Arnos K. S., Asher J. H., Jr, Baldwin C. T., Diehl S. R., Friedman T. B., Greenberg J., Grundfast K. M., Hoth C., Lalwani A. K. Locus heterogeneity for Waardenburg syndrome is predictive of clinical subtypes. Am J Hum Genet. 1994 Oct;55(4):728–737. [PMC free article] [PubMed] [Google Scholar]
- Ferré-D'Amaré A. R., Prendergast G. C., Ziff E. B., Burley S. K. Recognition by Max of its cognate DNA through a dimeric b/HLH/Z domain. Nature. 1993 May 6;363(6424):38–45. doi: 10.1038/363038a0. [DOI] [PubMed] [Google Scholar]
- Fisher D. E., Parent L. A., Sharp P. A. High affinity DNA-binding Myc analogs: recognition by an alpha helix. Cell. 1993 Feb 12;72(3):467–476. doi: 10.1016/0092-8674(93)90122-7. [DOI] [PubMed] [Google Scholar]
- Gregor P. D., Sawadogo M., Roeder R. G. The adenovirus major late transcription factor USF is a member of the helix-loop-helix group of regulatory proteins and binds to DNA as a dimer. Genes Dev. 1990 Oct;4(10):1730–1740. doi: 10.1101/gad.4.10.1730. [DOI] [PubMed] [Google Scholar]
- Hageman M. J., Delleman J. W. Heterogeneity in Waardenburg syndrome. Am J Hum Genet. 1977 Sep;29(5):468–485. [PMC free article] [PubMed] [Google Scholar]
- Hayashi K. PCR-SSCP: a simple and sensitive method for detection of mutations in the genomic DNA. PCR Methods Appl. 1991 Aug;1(1):34–38. doi: 10.1101/gr.1.1.34. [DOI] [PubMed] [Google Scholar]
- Hemesath T. J., Steingrímsson E., McGill G., Hansen M. J., Vaught J., Hodgkinson C. A., Arnheiter H., Copeland N. G., Jenkins N. A., Fisher D. E. microphthalmia, a critical factor in melanocyte development, defines a discrete transcription factor family. Genes Dev. 1994 Nov 15;8(22):2770–2780. doi: 10.1101/gad.8.22.2770. [DOI] [PubMed] [Google Scholar]
- Hodgkinson C. A., Moore K. J., Nakayama A., Steingrímsson E., Copeland N. G., Jenkins N. A., Arnheiter H. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 1993 Jul 30;74(2):395–404. doi: 10.1016/0092-8674(93)90429-t. [DOI] [PubMed] [Google Scholar]
- Hofstra R. M., Osinga J., Tan-Sindhunata G., Wu Y., Kamsteeg E. J., Stulp R. P., van Ravenswaaij-Arts C., Majoor-Krakauer D., Angrist M., Chakravarti A. A homozygous mutation in the endothelin-3 gene associated with a combined Waardenburg type 2 and Hirschsprung phenotype (Shah-Waardenburg syndrome). Nat Genet. 1996 Apr;12(4):445–447. doi: 10.1038/ng0496-445. [DOI] [PubMed] [Google Scholar]
- Hoth C. F., Milunsky A., Lipsky N., Sheffer R., Clarren S. K., Baldwin C. T. Mutations in the paired domain of the human PAX3 gene cause Klein-Waardenburg syndrome (WS-III) as well as Waardenburg syndrome type I (WS-I). Am J Hum Genet. 1993 Mar;52(3):455–462. [PMC free article] [PubMed] [Google Scholar]
- Liu X. Z., Newton V. E., Read A. P. Waardenburg syndrome type II: phenotypic findings and diagnostic criteria. Am J Med Genet. 1995 Jan 2;55(1):95–100. doi: 10.1002/ajmg.1320550123. [DOI] [PubMed] [Google Scholar]
- Miki T., Fleming T. P., Crescenzi M., Molloy C. J., Blam S. B., Reynolds S. H., Aaronson S. A. Development of a highly efficient expression cDNA cloning system: application to oncogene isolation. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5167–5171. doi: 10.1073/pnas.88.12.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima S., Sando I., Takahashi H., Hashida Y. Temporal bone histopathologic findings of Waardenburg's syndrome: a case report. Laryngoscope. 1992 May;102(5):563–567. doi: 10.1288/00005537-199205000-00016. [DOI] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Puffenberger E. G., Hosoda K., Washington S. S., Nakao K., deWit D., Yanagisawa M., Chakravart A. A missense mutation of the endothelin-B receptor gene in multigenic Hirschsprung's disease. Cell. 1994 Dec 30;79(7):1257–1266. doi: 10.1016/0092-8674(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Roman C., Matera A. G., Cooper C., Artandi S., Blain S., Ward D. C., Calame K. mTFE3, an X-linked transcriptional activator containing basic helix-loop-helix and zipper domains, utilizes the zipper to stabilize both DNA binding and multimerization. Mol Cell Biol. 1992 Feb;12(2):817–827. doi: 10.1128/mcb.12.2.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K. N., Dalal S. J., Desai M. P., Sheth P. N., Joshi N. C., Ambani L. M. White forelock, pigmentary disorder of irides, and long segment Hirschsprung disease: possible variant of Waardenburg syndrome. J Pediatr. 1981 Sep;99(3):432–435. doi: 10.1016/s0022-3476(81)80339-3. [DOI] [PubMed] [Google Scholar]
- Shibata K., Muraosa Y., Tomita Y., Tagami H., Shibahara S. Identification of a cis-acting element that enhances the pigment cell-specific expression of the human tyrosinase gene. J Biol Chem. 1992 Oct 15;267(29):20584–20588. [PubMed] [Google Scholar]
- Steingrímsson E., Moore K. J., Lamoreux M. L., Ferré-D'Amaré A. R., Burley S. K., Zimring D. C., Skow L. C., Hodgkinson C. A., Arnheiter H., Copeland N. G. Molecular basis of mouse microphthalmia (mi) mutations helps explain their developmental and phenotypic consequences. Nat Genet. 1994 Nov;8(3):256–263. doi: 10.1038/ng1194-256. [DOI] [PubMed] [Google Scholar]
- Tachibana M., Perez-Jurado L. A., Nakayama A., Hodgkinson C. A., Li X., Schneider M., Miki T., Fex J., Francke U., Arnheiter H. Cloning of MITF, the human homolog of the mouse microphthalmia gene and assignment to chromosome 3p14.1-p12.3. Hum Mol Genet. 1994 Apr;3(4):553–557. doi: 10.1093/hmg/3.4.553. [DOI] [PubMed] [Google Scholar]
- Tassabehji M., Newton V. E., Liu X. Z., Brady A., Donnai D., Krajewska-Walasek M., Murday V., Norman A., Obersztyn E., Reardon W. The mutational spectrum in Waardenburg syndrome. Hum Mol Genet. 1995 Nov;4(11):2131–2137. doi: 10.1093/hmg/4.11.2131. [DOI] [PubMed] [Google Scholar]
- Tassabehji M., Newton V. E., Read A. P. Waardenburg syndrome type 2 caused by mutations in the human microphthalmia (MITF) gene. Nat Genet. 1994 Nov;8(3):251–255. doi: 10.1038/ng1194-251. [DOI] [PubMed] [Google Scholar]
- Tassabehji M., Read A. P., Newton V. E., Harris R., Balling R., Gruss P., Strachan T. Waardenburg's syndrome patients have mutations in the human homologue of the Pax-3 paired box gene. Nature. 1992 Feb 13;355(6361):635–636. doi: 10.1038/355635a0. [DOI] [PubMed] [Google Scholar]
- WAARDENBURG P. J. A new syndrome combining developmental anomalies of the eyelids, eyebrows and nose root with pigmentary defects of the iris and head hair and with congenital deafness. Am J Hum Genet. 1951 Sep;3(3):195–253. [PMC free article] [PubMed] [Google Scholar]
- Wilkie A. O. The molecular basis of genetic dominance. J Med Genet. 1994 Feb;31(2):89–98. doi: 10.1136/jmg.31.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumoto K., Yokoyama K., Shibata K., Tomita Y., Shibahara S. Microphthalmia-associated transcription factor as a regulator for melanocyte-specific transcription of the human tyrosinase gene. Mol Cell Biol. 1994 Dec;14(12):8058–8070. doi: 10.1128/mcb.14.12.8058. [DOI] [PMC free article] [PubMed] [Google Scholar]