Abstract
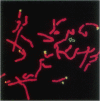
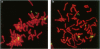
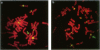
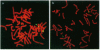
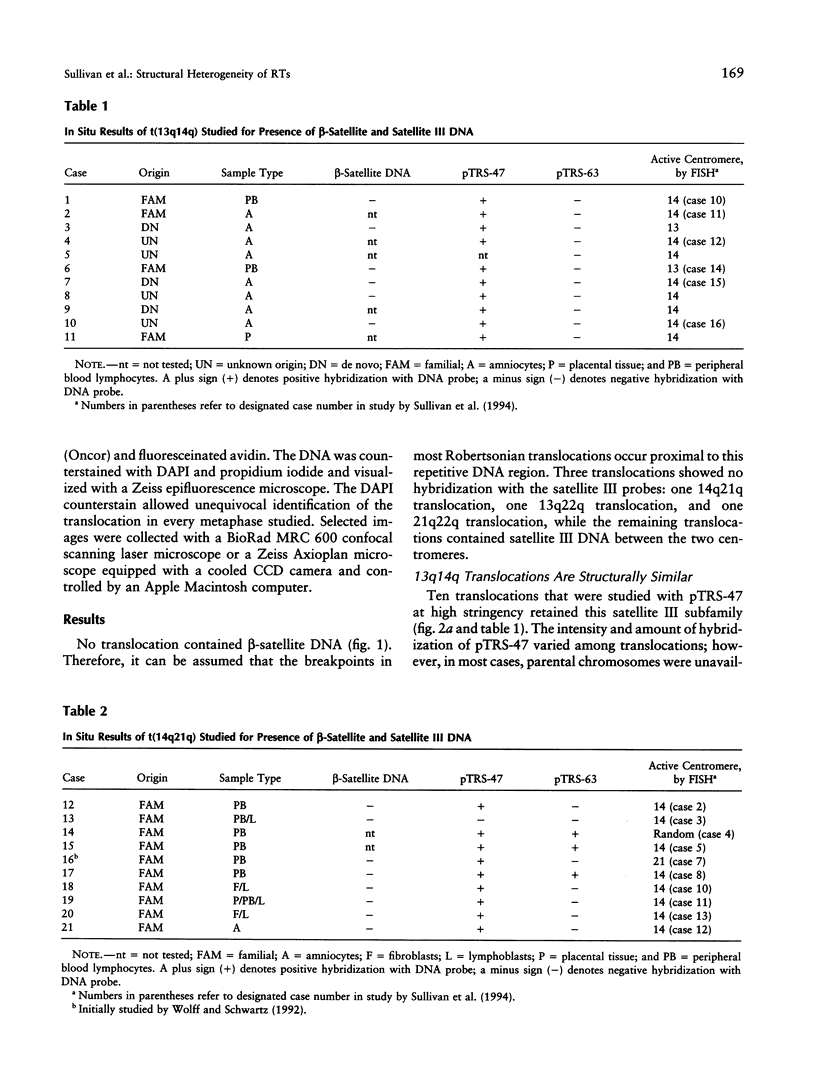
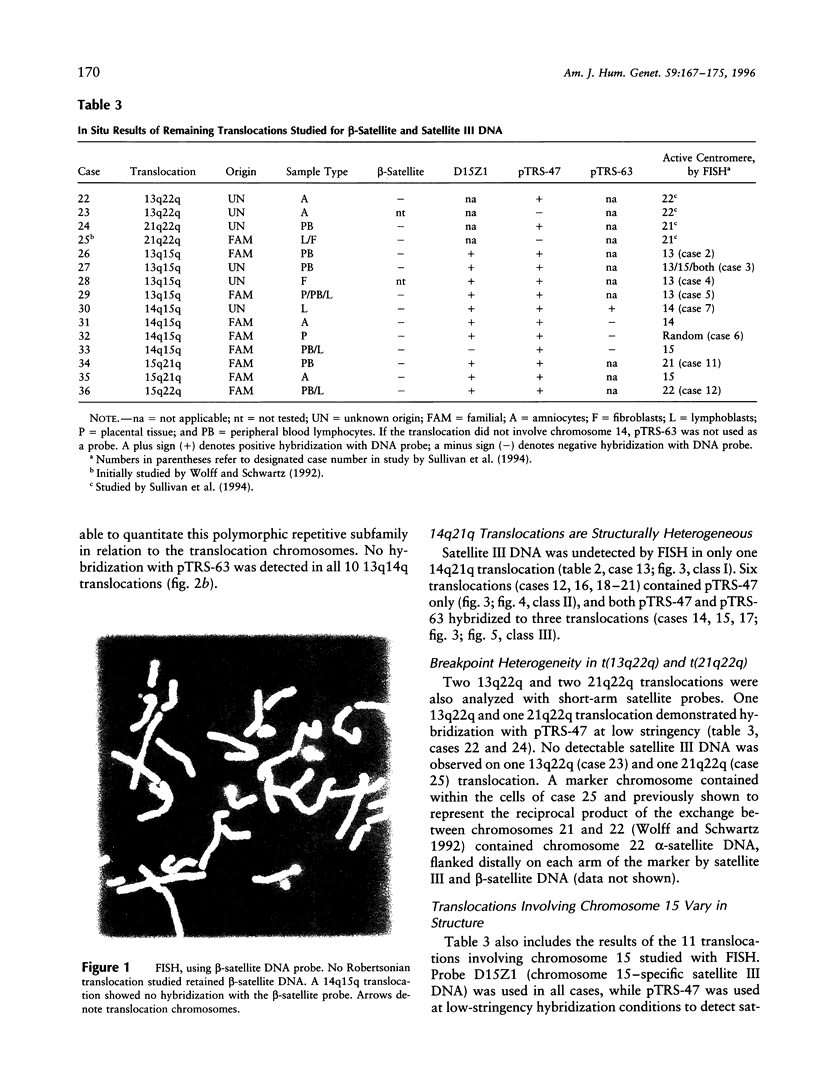
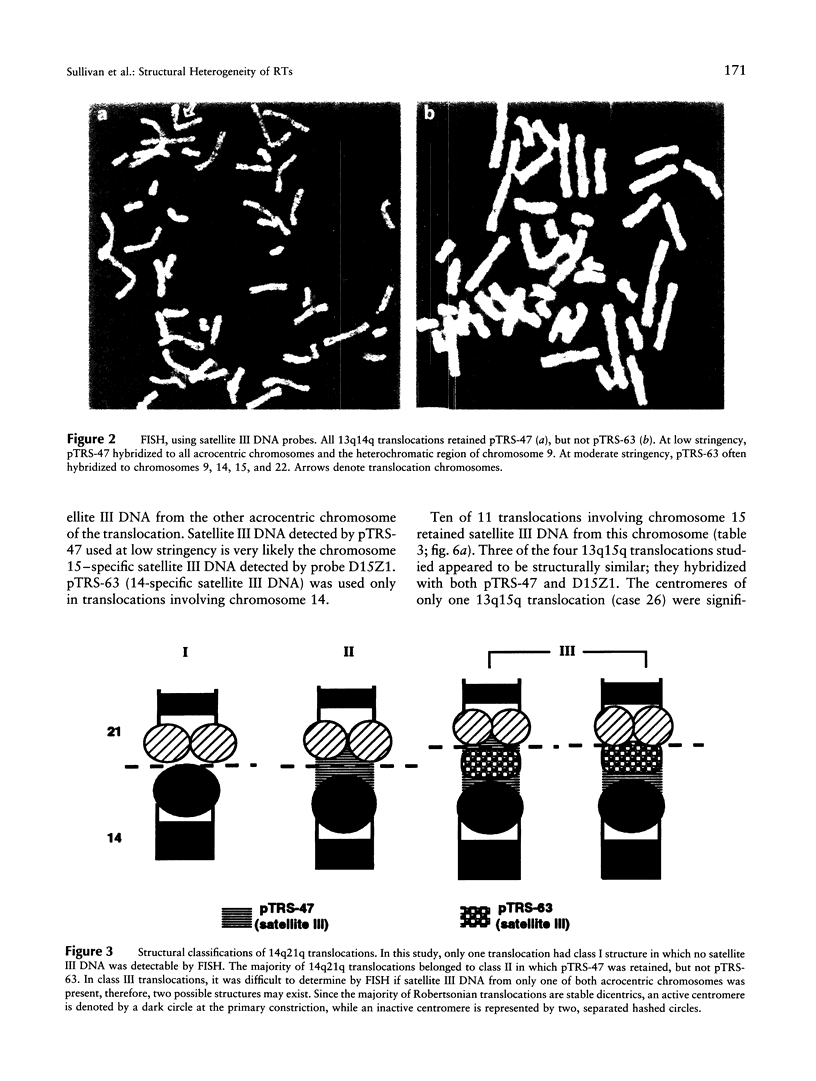
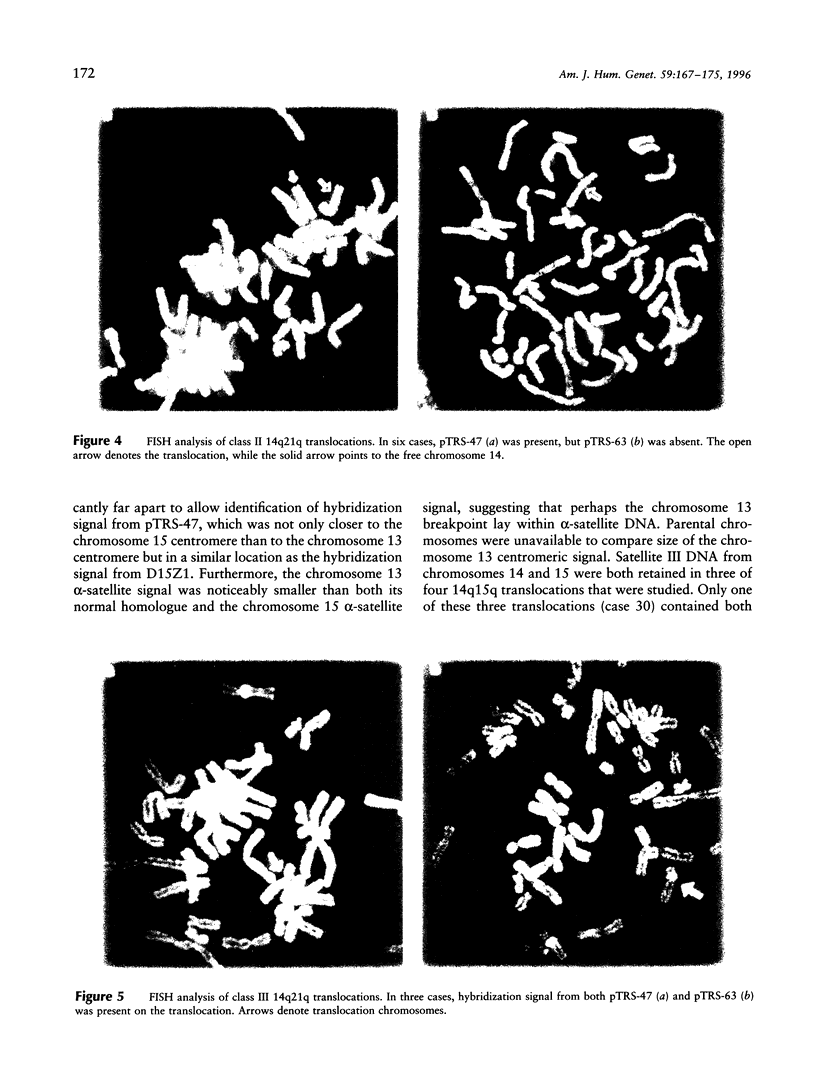
Most Robertsonian translocations are dicentric, suggesting that the location of chromosomal breaks leading to their formation occur in the acrocentric short arm. Previous cytogenetic and molecular cytogenetic studies have shown that few Robertsonian translocations retain ribosomal genes or beta-satellite DNA. Breakpoints in satellite III DNA, specifically between two chromosome 14-specific subfamilies, pTRS-47 and pTRS-63, have been indicated for most of the dicentric 14q21q and 13q14q translocations that have been studied. We have analyzed the structure of 36 dicentric translocations, using several repetitive DNA probes that localize to the acrocentric short arm. The majority of the translocations retained satellite III DNA, while others proved variable in structure. Of 10 14q21q translocations analyzed, satellite III DNA was undetected in 1; 6 retained one satellite III DNA subfamily, pTRS-47; and 3 appeared to contain two 14-specific satellite III DNA sub-families, pTRS-47 and pTRS-63. In 10/11 translocations involving chromosome 15, the presence of satellite III DNA was observed. Our results show that various regions of the acrocentric short arm, and, particularly, satellite III DNA sequences, are involved in the formation of Robertsonian translocations.
Full text
PDF

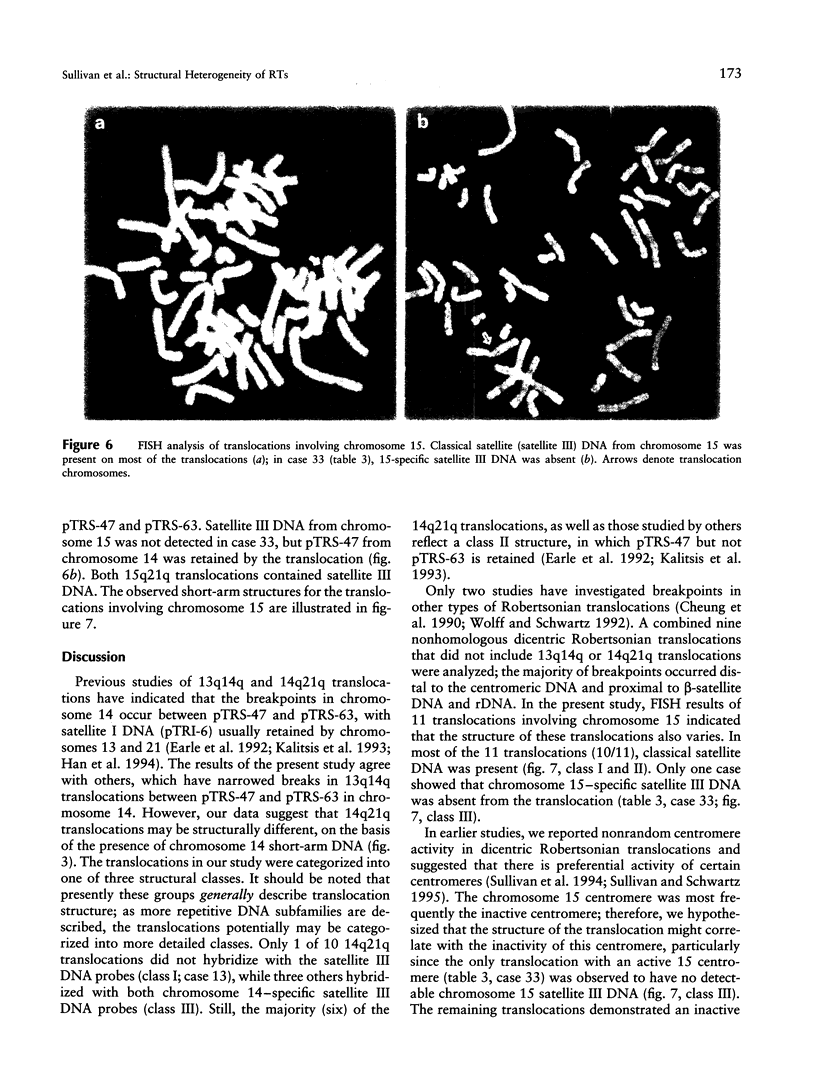
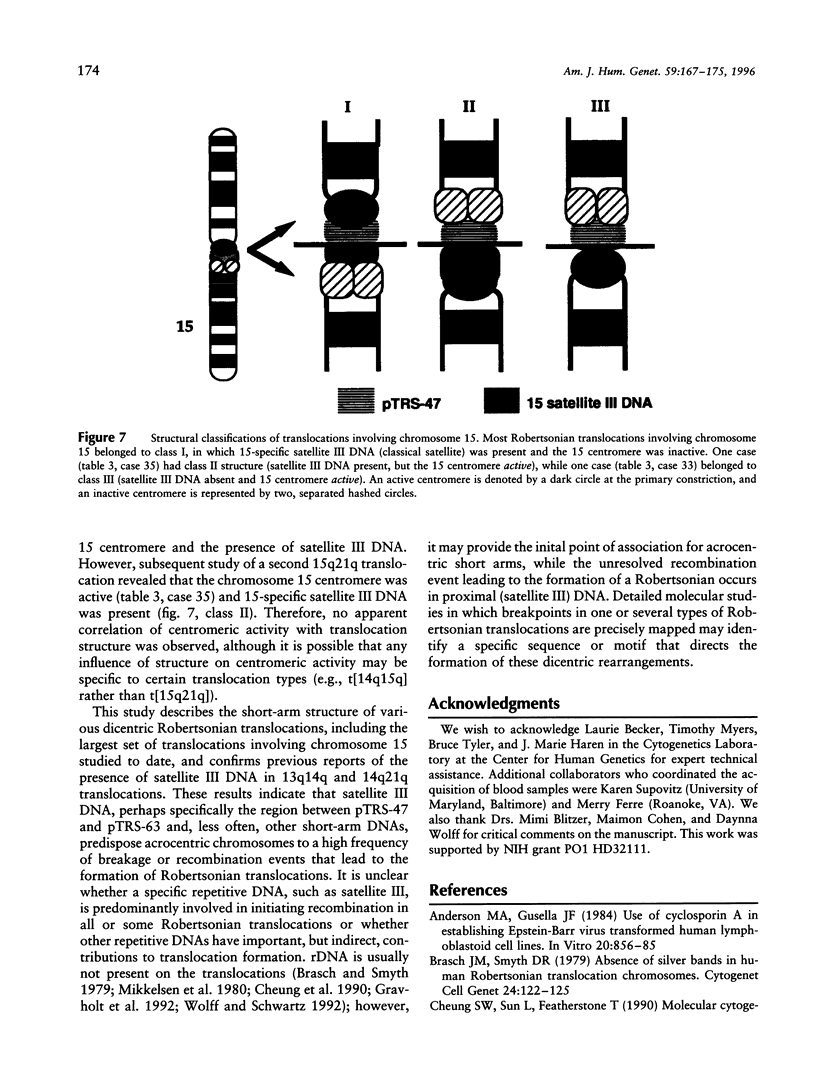

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. A., Gusella J. F. Use of cyclosporin A in establishing Epstein-Barr virus-transformed human lymphoblastoid cell lines. In Vitro. 1984 Nov;20(11):856–858. doi: 10.1007/BF02619631. [DOI] [PubMed] [Google Scholar]
- Brasch J. M., Smyth D. R. Absence of silver bands in human Robertsonian translocation chromosomes. Cytogenet Cell Genet. 1979;24(2):122–125. doi: 10.1159/000131365. [DOI] [PubMed] [Google Scholar]
- Cheung S. W., Sun L., Featherstone T. Molecular cytogenetic evidence to characterize breakpoint regions in Robertsonian translocations. Cytogenet Cell Genet. 1990;54(3-4):97–102. doi: 10.1159/000132970. [DOI] [PubMed] [Google Scholar]
- Choo K. H., Earle E., McQuillan C. A homologous subfamily of satellite III DNA on human chromosomes 14 and 22. Nucleic Acids Res. 1990 Oct 11;18(19):5641–5648. doi: 10.1093/nar/18.19.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo K. H., Earle E., Vissel B., Filby R. G. Identification of two distinct subfamilies of alpha satellite DNA that are highly specific for human chromosome 15. Genomics. 1990 Jun;7(2):143–151. doi: 10.1016/0888-7543(90)90534-2. [DOI] [PubMed] [Google Scholar]
- Choo K. H., Earle E., Vissel B., Kalitsis P. A chromosome 14-specific human satellite III DNA subfamily that shows variable presence on different chromosomes 14. Am J Hum Genet. 1992 Apr;50(4):706–716. [PMC free article] [PubMed] [Google Scholar]
- Choo K. H., Vissel B., Brown R., Filby R. G., Earle E. Homologous alpha satellite sequences on human acrocentric chromosomes with selectivity for chromosomes 13, 14 and 21: implications for recombination between nonhomologues and Robertsonian translocations. Nucleic Acids Res. 1988 Feb 25;16(4):1273–1284. doi: 10.1093/nar/16.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel A., Lam-Po-Tang P. R. Structure and inheritance of some heterozygous Robertsonian translocation in man. J Med Genet. 1976 Oct;13(5):381–388. doi: 10.1136/jmg.13.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earle E., Shaffer L. G., Kalitsis P., McQuillan C., Dale S., Choo K. H. Identification of DNA sequences flanking the breakpoint of human t(14q21q) Robertsonian translocations. Am J Hum Genet. 1992 Apr;50(4):717–724. [PMC free article] [PubMed] [Google Scholar]
- Gosden J. R., Lawrie S. S., Gosden C. M. Satellite DNA sequences in the human acrocentric chromosomes: information from translocations and heteromorphisms. Am J Hum Genet. 1981 Mar;33(2):243–251. [PMC free article] [PubMed] [Google Scholar]
- Gravholt C. H., Friedrich U., Caprani M., Jørgensen A. L. Breakpoints in Robertsonian translocations are localized to satellite III DNA by fluorescence in situ hybridization. Genomics. 1992 Dec;14(4):924–930. doi: 10.1016/s0888-7543(05)80113-2. [DOI] [PubMed] [Google Scholar]
- Guichaoua M. R., Devictor M., Hartung M., Luciani J. M., Stahl A. Random acrocentric bivalent associations in human pachytene spermatocytes. Molecular implications in the occurrence of Robertsonian translocations. Cytogenet Cell Genet. 1986;42(4):191–197. doi: 10.1159/000132277. [DOI] [PubMed] [Google Scholar]
- Han J. Y., Choo K. H., Shaffer L. G. Molecular cytogenetic characterization of 17 rob(13q14q) Robertsonian translocations by FISH, narrowing the region containing the breakpoints. Am J Hum Genet. 1994 Nov;55(5):960–967. [PMC free article] [PubMed] [Google Scholar]
- Higgins M. J., Wang H. S., Shtromas I., Haliotis T., Roder J. C., Holden J. J., White B. N. Organization of a repetitive human 1.8 kb KpnI sequence localized in the heterochromatin of chromosome 15. Chromosoma. 1985;93(1):77–86. doi: 10.1007/BF01259449. [DOI] [PubMed] [Google Scholar]
- Kalitsis P., Earle E., Vissel B., Shaffer L. G., Choo K. H. A chromosome 13-specific human satellite I DNA subfamily with minor presence on chromosome 21: further studies on Robertsonian translocations. Genomics. 1993 Apr;16(1):104–112. doi: 10.1006/geno.1993.1147. [DOI] [PubMed] [Google Scholar]
- MOORHEAD P. S., NOWELL P. C., MELLMAN W. J., BATTIPS D. M., HUNGERFORD D. A. Chromosome preparations of leukocytes cultured from human peripheral blood. Exp Cell Res. 1960 Sep;20:613–616. doi: 10.1016/0014-4827(60)90138-5. [DOI] [PubMed] [Google Scholar]
- Mikkelsen M., Basli A., Poulsen H. Nucleolus organizer regions in translocations involving acrocentric chromosomes. Cytogenet Cell Genet. 1980;26(1):14–21. doi: 10.1159/000131416. [DOI] [PubMed] [Google Scholar]
- Niebuhr E. Dicentric and monocentric Robertsonian translocations in man. Humangenetik. 1972;16(3):217–226. doi: 10.1007/BF00273467. [DOI] [PubMed] [Google Scholar]
- Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971 Oct 30;2(7731):971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- Sullivan B. A., Schwartz S. Identification of centromeric antigens in dicentric Robertsonian translocations: CENP-C and CENP-E are necessary components of functional centromeres. Hum Mol Genet. 1995 Dec;4(12):2189–2197. doi: 10.1093/hmg/4.12.2189. [DOI] [PubMed] [Google Scholar]
- Sullivan B. A., Wolff D. J., Schwartz S. Analysis of centromeric activity in Robertsonian translocations: implications for a functional acrocentric hierarchy. Chromosoma. 1994 Dec;103(7):459–467. doi: 10.1007/BF00337384. [DOI] [PubMed] [Google Scholar]
- Therman E., Susman B., Denniston C. The nonrandom participation of human acrocentric chromosomes in Robertsonian translocations. Ann Hum Genet. 1989 Jan;53(Pt 1):49–65. doi: 10.1111/j.1469-1809.1989.tb01121.x. [DOI] [PubMed] [Google Scholar]
- Trowell H. E., Nagy A., Vissel B., Choo K. H. Long-range analyses of the centromeric regions of human chromosomes 13, 14 and 21: identification of a narrow domain containing two key centromeric DNA elements. Hum Mol Genet. 1993 Oct;2(10):1639–1649. doi: 10.1093/hmg/2.10.1639. [DOI] [PubMed] [Google Scholar]
- Wolff D. J., Schwartz S. Characterization of Robertsonian translocations by using fluorescence in situ hybridization. Am J Hum Genet. 1992 Jan;50(1):174–181. [PMC free article] [PubMed] [Google Scholar]