Abstract
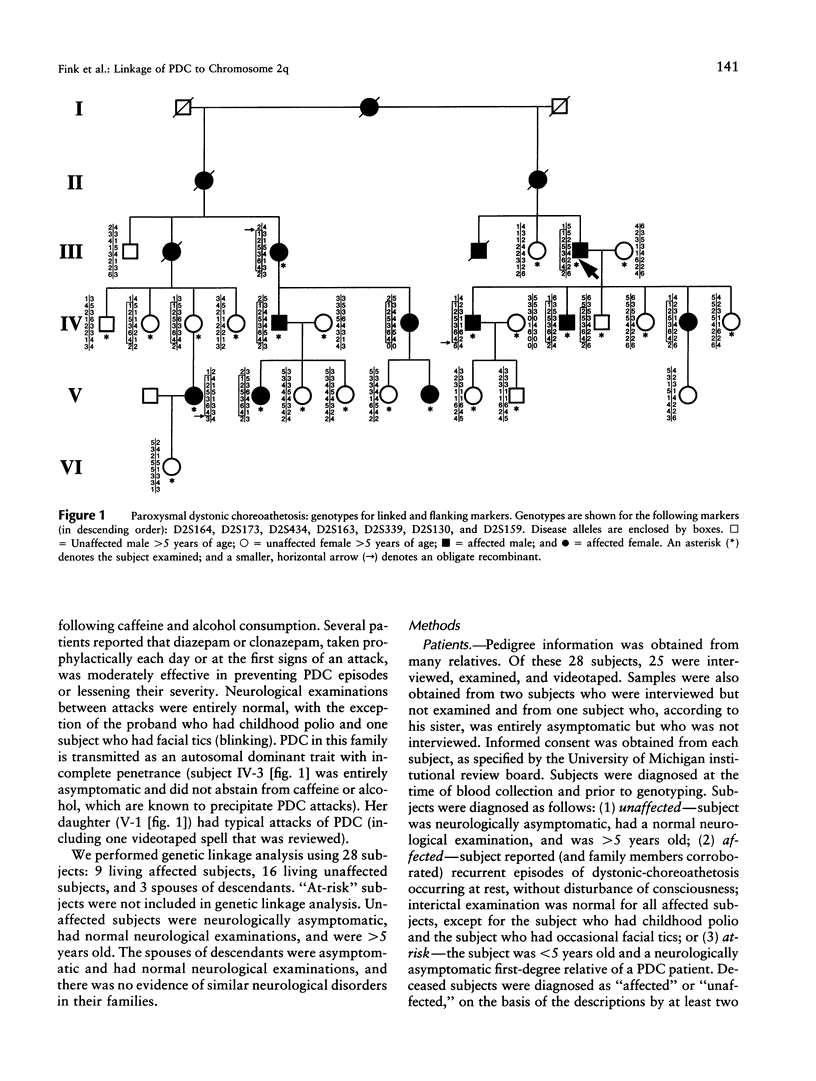
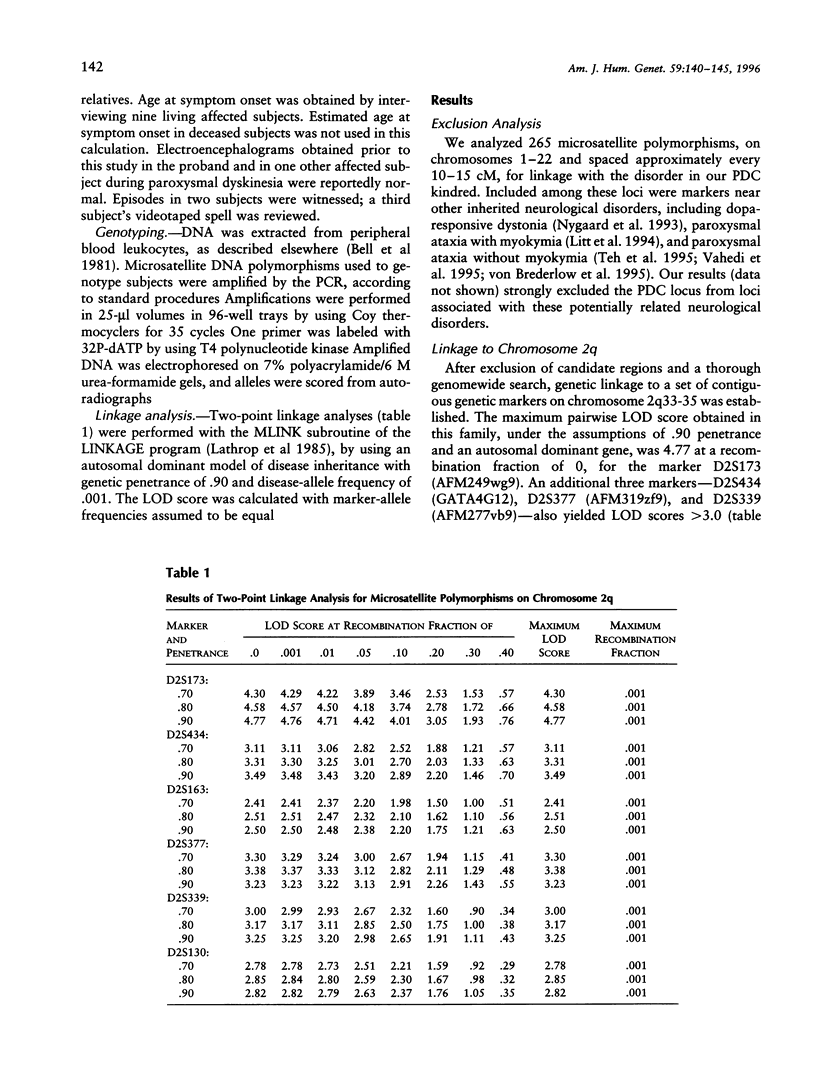
Paroxysmal dystonic choreoathetosis (PDC) is characterized by attacks of involuntary movements that last up to several hours and occur at rest both spontaneously and following caffeine or alcohol consumption. We analyzed a Polish-American kindred with autosomal dominant PDC and identified tight linkage between the disorder and microsatellite markers on chromosome 2q (maximum two-point LOD score 4.77; recombination fraction 0). Our results clearly establish the existence of a locus for autosomal dominant PDC on distal chromosome 2q. The fact that three other paroxysmal neurological disorders (periodic ataxia with myokymia and hypo- and hyperkalemic periodic paralysis) are due to mutation in ion-channel genes raises the possibility that PDC is also due to an ion-channel gene mutation. It is noteworthy that a cluster of sodium-channel genes is located on distal chromosome 2q, near the PDC locus. Identifying the PDC locus on chromosome 2q will facilitate discovery of the PDC gene and enable investigators to determine whether PDC is genetically homogeneous and whether other paroxysmal movement disorders are also genetically linked to the PDC locus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auburger G., Ratzlaff T., Lunkes A., Nelles H. W., Leube B., Binkofski F., Kugel H., Heindel W., Seitz R., Benecke R. A gene for autosomal dominant paroxysmal choreoathetosis/spasticity (CSE) maps to the vicinity of a potassium channel gene cluster on chromosome 1p, probably within 2 cM between D1S443 and D1S197. Genomics. 1996 Jan 1;31(1):90–94. doi: 10.1006/geno.1996.0013. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Karam J. H., Rutter W. J. Polymorphic DNA region adjacent to the 5' end of the human insulin gene. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5759–5763. doi: 10.1073/pnas.78.9.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne D. L., Gancher S. T., Nutt J. G., Brunt E. R., Smith E. A., Kramer P., Litt M. Episodic ataxia/myokymia syndrome is associated with point mutations in the human potassium channel gene, KCNA1. Nat Genet. 1994 Oct;8(2):136–140. doi: 10.1038/ng1094-136. [DOI] [PubMed] [Google Scholar]
- Byrne E., White O., Cook M. Familial dystonic choreoathetosis with myokymia; a sleep responsive disorder. J Neurol Neurosurg Psychiatry. 1991 Dec;54(12):1090–1092. doi: 10.1136/jnnp.54.12.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirkiran M., Jankovic J. Paroxysmal dyskinesias: clinical features and classification. Ann Neurol. 1995 Oct;38(4):571–579. doi: 10.1002/ana.410380405. [DOI] [PubMed] [Google Scholar]
- Fahn S. Paroxysmal myoclonic dystonia with vocalisations. J Neurol Neurosurg Psychiatry. 1987 Jan;50(1):117–118. doi: 10.1136/jnnp.50.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine B., Vale-Santos J., Jurkat-Rott K., Reboul J., Plassart E., Rime C. S., Elbaz A., Heine R., Guimarães J., Weissenbach J. Mapping of the hypokalaemic periodic paralysis (HypoPP) locus to chromosome 1q31-32 in three European families. Nat Genet. 1994 Mar;6(3):267–272. doi: 10.1038/ng0394-267. [DOI] [PubMed] [Google Scholar]
- Gyapay G., Morissette J., Vignal A., Dib C., Fizames C., Millasseau P., Marc S., Bernardi G., Lathrop M., Weissenbach J. The 1993-94 Généthon human genetic linkage map. Nat Genet. 1994 Jun;7(2 Spec No):246–339. doi: 10.1038/ng0694supp-246. [DOI] [PubMed] [Google Scholar]
- Jurkat-Rott K., Lehmann-Horn F., Elbaz A., Heine R., Gregg R. G., Hogan K., Powers P. A., Lapie P., Vale-Santos J. E., Weissenbach J. A calcium channel mutation causing hypokalemic periodic paralysis. Hum Mol Genet. 1994 Aug;3(8):1415–1419. doi: 10.1093/hmg/3.8.1415. [DOI] [PubMed] [Google Scholar]
- Lance J. W. Familial paroxysmal dystonic choreoathetosis and its differentiation from related syndromes. Ann Neurol. 1977 Oct;2(4):285–293. doi: 10.1002/ana.410020405. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Multilocus linkage analysis in humans: detection of linkage and estimation of recombination. Am J Hum Genet. 1985 May;37(3):482–498. [PMC free article] [PubMed] [Google Scholar]
- Litt M., Kramer P., Browne D., Gancher S., Brunt E. R., Root D., Phromchotikul T., Dubay C. J., Nutt J. A gene for episodic ataxia/myokymia maps to chromosome 12p13. Am J Hum Genet. 1994 Oct;55(4):702–709. [PMC free article] [PubMed] [Google Scholar]
- Litt M., Luty J., Kwak M., Allen L., Magenis R. E., Mandel G. Localization of a human brain sodium channel gene (SCN2A) to chromosome 2. Genomics. 1989 Aug;5(2):204–208. doi: 10.1016/0888-7543(89)90047-5. [DOI] [PubMed] [Google Scholar]
- Malo M. S., Blanchard B. J., Andresen J. M., Srivastava K., Chen X. N., Li X., Jabs E. W., Korenberg J. R., Ingram V. M. Localization of a putative human brain sodium channel gene (SCN1A) to chromosome band 2q24. Cytogenet Cell Genet. 1994;67(3):178–186. doi: 10.1159/000133818. [DOI] [PubMed] [Google Scholar]
- Malo M. S., Srivastava K., Andresen J. M., Chen X. N., Korenberg J. R., Ingram V. M. Targeted gene walking by low stringency polymerase chain reaction: assignment of a putative human brain sodium channel gene (SCN3A) to chromosome 2q24-31. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2975–2979. doi: 10.1073/pnas.91.8.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchey A. I., McKenna-Yasek D., Cros D., Worthen H. G., Kuncl R. W., DeSilva S. M., Cornblath D. R., Gusella J. F., Brown R. H., Jr Novel mutations in families with unusual and variable disorders of the skeletal muscle sodium channel. Nat Genet. 1992 Oct;2(2):148–152. doi: 10.1038/ng1092-148. [DOI] [PubMed] [Google Scholar]
- Nakano T., Kondo K., Oguchi K., Yanagisawa N., Nakano T. [A late onset case of paroxysmal dystonic choreoathetosis induced by caffeine and aminophylline]. Rinsho Shinkeigaku. 1983 Mar;23(3):199–202. [PubMed] [Google Scholar]
- Nygaard T. G., Wilhelmsen K. C., Risch N. J., Brown D. L., Trugman J. M., Gilliam T. C., Fahn S., Weeks D. E. Linkage mapping of dopa-responsive dystonia (DRD) to chromosome 14q. Nat Genet. 1993 Dec;5(4):386–391. doi: 10.1038/ng1293-386. [DOI] [PubMed] [Google Scholar]
- Ptáek L. J., Tawil R., Griggs R. C., Meola G., McManis P., Barohn R. J., Mendell J. R., Harris C., Spitzer R., Santiago F. Sodium channel mutations in acetazolamide-responsive myotonia congenita, paramyotonia congenita, and hyperkalemic periodic paralysis. Neurology. 1994 Aug;44(8):1500–1503. doi: 10.1212/wnl.44.8.1500. [DOI] [PubMed] [Google Scholar]
- Richards R. N., Barnett H. J. Paroxysmal dystonic choreoathetosis. A family study and review of the literature. Neurology. 1968 May;18(5):461–469. doi: 10.1212/wnl.18.5.461. [DOI] [PubMed] [Google Scholar]
- Rojas C. V., Wang J. Z., Schwartz L. S., Hoffman E. P., Powell B. R., Brown R. H., Jr A Met-to-Val mutation in the skeletal muscle Na+ channel alpha-subunit in hyperkalaemic periodic paralysis. Nature. 1991 Dec 5;354(6352):387–389. doi: 10.1038/354387a0. [DOI] [PubMed] [Google Scholar]
- Stoffel M., Espinosa R., 3rd, Powell K. L., Philipson L. H., Le Beau M. M., Bell G. I. Human G-protein-coupled inwardly rectifying potassium channel (GIRK1) gene (KCNJ3): localization to chromosome 2 and identification of a simple tandem repeat polymorphism. Genomics. 1994 May 1;21(1):254–256. doi: 10.1006/geno.1994.1253. [DOI] [PubMed] [Google Scholar]
- Teh B. T., Silburn P., Lindblad K., Betz R., Boyle R., Schalling M., Larsson C. Familial periodic cerebellar ataxia without myokymia maps to a 19-cM region on 19p13. Am J Hum Genet. 1995 Jun;56(6):1443–1449. [PMC free article] [PubMed] [Google Scholar]
- Vahedi K., Joutel A., Van Bogaert P., Ducros A., Maciazeck J., Bach J. F., Bousser M. G., Tournier-Lasserve E. A gene for hereditary paroxysmal cerebellar ataxia maps to chromosome 19p. Ann Neurol. 1995 Mar;37(3):289–293. doi: 10.1002/ana.410370304. [DOI] [PubMed] [Google Scholar]
- von Brederlow B., Hahn A. F., Koopman W. J., Ebers G. C., Bulman D. E. Mapping the gene for acetazolamide responsive hereditary paryoxysmal cerebellar ataxia to chromosome 19p. Hum Mol Genet. 1995 Feb;4(2):279–284. doi: 10.1093/hmg/4.2.279. [DOI] [PubMed] [Google Scholar]


