Abstract
Capsaicin, a pungent constituent from red chilli peppers, activates sensory nerve fibres via transient receptor potential vanilloid receptors type 1 (TRPV1) to release neuropeptides like calcitonin gene-related peptide (CGRP) and substance P. Capsaicin-sensitive nerves are widely distributed in human and porcine vasculature. In this study, we examined the mechanism of capsaicin-induced relaxations, with special emphasis on the role of CGRP, using various pharmacological tools. Segments of human and porcine proximal and distal coronary arteries, as well as cranial arteries, were mounted in organ baths. Concentration response curves to capsaicin were constructed in the absence or presence of the CGRP receptor antagonist olcegepant (BIBN4096BS, 1 μM), the neurokinin NK1 receptor antagonist L-733060 (0.5 μM), the voltage-sensitive calcium channel blocker ruthenium red (100 μM), the TRPV1 receptor antagonist capsazepine (5 μM), the nitric oxide synthetase inhibitor Nω-nitro-l-arginine methyl ester HCl (l-NAME; 100 μM), the gap junction blocker 18α-glycyrrhetinic acid (10 μM), as well as the RhoA kinase inhibitor Y-27632 (1 μM). Further, we also used the K+ channel inhibitors 4-aminopyridine (1 mM), charybdotoxin (0.5 μM) + apamin (0.1 μM) and iberiotoxin (0.5 μM) + apamin (0.1 μM). The role of the endothelium was assessed by endothelial denudation in distal coronary artery segments. In distal coronary artery segments, we also measured levels of cyclic adenosine monophosphate (cAMP) after exposure to capsaicin, and in human segments, we also assessed the amount of CGRP released in the organ bath fluid after exposure to capsaicin. Capsaicin evoked concentration-dependent relaxant responses in precontracted arteries, but none of the above-mentioned inhibitors did affect these relaxations. There was no increase in the cAMP levels after exposure to capsaicin, unlike after (exogenously administered) α-CGRP. Interestingly, there were significant increases in CGRP levels after exposure to vehicle (ethanol) as well as capsaicin, although this did not induce relaxant responses. In conclusion, the capsaicin-induced relaxations of the human and porcine distal coronary arteries are not mediated by CGRP, NK1, NO, vanilloid receptors, voltage-sensitive calcium channels, K+ channels or cAMP-mediated mechanisms. Therefore, these relaxant responses to capsaicin are likely to be attributed to a non-specific, CGRP-independent mechanism.
Keywords: Capsaicin, CGRP, Human coronary artery, Human meningeal artery, Porcine coronary artery
Introduction
Capsaicin, a pungent constituent of red pepper, is known to activate sensory C-fibres via transient receptor potential vanilloid receptors type 1 (TRPV1; Caterina et al. 1997; Gunthorpe et al. 2002; Szallasi 2006), which are nonselective cation channels. Activation of these channels increases influx of mono- and divalent cations, which leads to an increase in the intracellular Ca2+ concentrations. Consequently, an array of neuropeptides like calcitonin gene-related peptide (CGRP), substance P and neurokinin A are released (Franco-Cereceda et al. 1988; Mitchell et al. 1995). These neuropeptides play a role in the regulation of normal vascular smooth muscle tone and are also implicated in several pathological conditions like ischemic preconditioning (Chai et al. 2006), preeclampsia (Dong et al. 2005) and migraine (Arulmani et al. 2004b). In the pathophysiology of migraine, vasodilatation of cranial blood vessels, especially extracerebral intracranial blood vessels, which are richly innervated by nerves containing a number of peptides such as CGRP, seems pivotal (Arulmani et al. 2006). Vasodilatation of these intracranial arteries leads to activation of nociceptors, which stimulate the pain centres in the brain (Goadsby et al. 2002). Involvement of CGRP in the pathophysiology of migraine is further strengthened by the observation that olcegepant (BIBN4096BS, Doods et al. 2000), a CGRP receptor antagonist, is effective in the acute treatment of migraine attacks (Olesen et al. 2004). Capsaicin has been widely used in various in vivo models of migraine to induce cranial vasodilatation, which is attributed to endogenous release of neuropeptides, especially CGRP (Akerman et al. 2003; Arulmani et al. 2004a; Gupta et al. 2006a). The involvement of CGRP is further substantiated in all these in vivo studies by the fact that the capsaicin-induced vascular responses are all amenable to blockade with CGRP receptor antagonists. Repeated capsaicin challenges are reported to deplete a number of neurotransmitters, including CGRP in various animal models (Tang et al. 1997; Tang et al. 1999; Zhou et al. 2002). In addition to the in vivo models, also in vitro vascular models involving meningeal (Gupta et al. 2006b) and coronary (Edvinsson et al. 2002; Gupta et al. 2006c) arteries have been used in migraine research, but vasorelaxation in these models was induced by exogenously administered CGRP. Several major vascular beds, including the meningeal and coronary (Franco-Cereceda 1988), are richly innervated with capsaicin-sensitive sensory fibres containing CGRP (Edvinsson et al. 1987). Meningeal blood vessels have been shown to be involved in the plasma protein extravasation in rat dura mater (Delepine and Aubineau 1997; Seabrook et al. 1996), a model of neurogenic inflammation, one of the putative mechanisms in migraine pathophysiology. In this model, capsaicin pre-treatment has been shown to block plasma protein extravasation, again by depleting neuropeptides (Delepine and Aubineau 1997). Therefore, it is of interest to investigate the release of endogenous CGRP in various blood vessels and capsaicin seems most appropriate for such studies.
Interestingly, very few studies have investigated capsaicin-induced relaxations in human isolated blood vessels (Franco-Cereceda 1991a; Franco-Cereceda et al. 1987). Based on CGRP-like immunoreactivity, it was claimed that capsaicin-induced relaxations are mediated by CGRP (Franco-Cereceda 1991b), but no CGRP receptor antagonists were used to unequivocally demonstrate the role of CGRP in these responses. Therefore, we were interested in studying capsaicin-mediated relaxations in human and porcine isolated arteries as a model to study endogenous release of CGRP in view of its relevance in the pathophysiology of migraine. Remarkably, some recent studies indicate that capsaicin-induced relaxations are mediated by non-CGRP-mediated mechanisms in guinea pig ileum, rabbit coronary arteries and equine tracheal smooth muscle preparations (Fujimoto et al. 2006; Yeon et al. 2001; Zhu et al. 1997), which is contrary to the observation that capsaicin-induced relaxations are mediated principally by CGRP. In these studies, relaxant responses to capsaicin were mainly attributed to different Ca2+-activated K+ channels. Therefore, in the present study, we tried to characterise capsaicin-induced relaxant response in human and porcine vessels, with special emphasis on the role of CGRP, and also investigated other mechanisms, using different pharmacological tools.
Materials and methods
Tissue preparation
The study protocol was approved by the ethical committee of Erasmus MC. Human coronary artery was obtained from ‘heart-beating’ organ donors (13 men, 19 women; 13–65 years) who died due to non-cardiac disorders. The hearts were provided by the Heart Valve Bank, Rotterdam, The Netherlands, after donor mediation by Bio Implant Services Foundation/Eurotransplant Foundation (Leiden, The Netherlands). The meningeal arteries were obtained from patients (four men, six women; 41–75 years) undergoing craniotomy at the neurosurgical unit (n = 6) or from the Department of Pathology (postmortem, n = 4) within 24 h of death, at Erasmus MC, Rotterdam. During the surgical procedure or autopsy, the dura mater together with a small piece of the meningeal artery was cut and placed in a plastic container filled with ice-cold (0–4°C), physiological salt solution. The artery segment was immediately transported to the laboratory and placed in cold oxygenated Krebs bicarbonate solution of the following composition (mM): NaCl 119, KCl 4.7, CaCl2 1.25, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25 and glucose 11.1; pH 7.4. Excess tissue was removed. The meningeal artery (internal diameter of 350–800 μm) was used the same day or stored overnight in cold oxygenated modified Krebs solution and used the following day. Porcine hearts and heads (pigs of either sex; 6–12 months of age) were collected from a local slaughterhouse. Porcine basilar (internal diameter of 150–200 μm) as well as meningeal (internal diameter of 100–250 μm) arteries were dissected out from the skull and were placed in a cold oxygenated Krebs solution as described earlier for human meningeal arteries. In the laboratory, both human and porcine proximal (internal diameter, 2–3.5 mm) and distal (internal diameter, 200–600 μm) coronary arteries were dissected out of the right ventricle; distal coronary arteries were dissected with the aid of a microscope and stored in bicarbonate solution of following composition (mM): NaCl 118, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25 and glucose 11.1; pH 7.4.
Functional studies
Both human and porcine proximal coronary artery segments (3–4 mm length) were suspended with the help of stainless steel hooks in 15-ml organ baths with a pretension of 15 mN (optimal tension shown in earlier experiments). Distal coronary and cranial artery segments were cut into ring segments of 1–2 mm length and were mounted in Mulvany myographs between two parallel titanium wires with a tension normalised to 90% of l100 (distance when transmural pressure equals 100 mmHg), thus achieving optimal conditions for active force development (Nyborg et al. 1987). Vessels were allowed to equilibrate for 30 min in Krebs solution at 37°C, a similar equilibration period was repeated after each physical or pharmacological challenge. In case of human arteries, the cyclo-oxygenase inhibitor indomethacin (0.1 μM) was added to prevent prostaglandin synthesis during the whole experimental protocol. Two successive challenges to KCl (30 mM) were performed to test the reproducibility of responses. Endothelial integrity was assessed by observing relaxation to substance P (10 nM) after precontraction with U46619 (9,11-dideoxy-11α, 9α-epoxymethano-prostaglandin F2α; 10 nM–1 μM), followed by washout of the agonists. KCl (18–30 mM, except where higher concentrations are indicated) or U46619 (10 nM–1 μM), in case of the K+ channel blockers, was used to obtain a stable contraction plateau of around 60–75% of the maximal contraction reached with KCl (100 mM). Subsequently, capsaicin was added in a cumulative manner in log steps. The different antagonists were used to antagonise capsaicin responses, and the concentration of each of these antagonists was decided on the basis of highest concentration in the literature unless mentioned otherwise. Concentration response curves were constructed in the absence or presence of the CGRP receptor antagonists olcegepant (1 μM; Gupta et al. 2006b,c) and CGRP8–37 (10 μM; Gupta et al. 2006b,c), the neurokinin NK1 receptor antagonist L-733060 (0.5 μM; Seabrook et al. 1996), the voltage-sensitive calcium channel blocker ruthenium red (100 μM, 45–90 mM KCl was required to get a similar precontraction as in the corresponding control segments; Franco-Cereceda and Rudehill 1989), the nitric oxide synthetase inhibitor Nω-nitro-l-arginine methyl ester HCl (l-NAME; 100 μM; Batenburg et al. 2004a,b), as well as the vanilloid receptor antagonist capsazepine (5 μM; Fujimoto et al. 2006; Gazzieri et al. 2006). At still higher concentrations of capsazepine (>5 μM) and L-733006 (>0.5 μM), even higher concentrations of preconstricting agents could not produce a workable precontraction.
We also investigated the role of different K+ channels by using various inhibitors, 4-aminopyridine (1 mM; Fujimoto et al. 2006; Yeon et al. 2001), a voltage-dependent K+ channel (Kv) blocker and charybdotoxin (0.5 μM), a blocker of Ca2+-dependent K+ channels for large conductance (BKCa) and intermediate conductance (IKCa), in combination with apamin (0.1 μM), a blocker of small-conductance Ca2+-dependent K+ channels (SKCa; Batenburg et al. 2004b; Fujimoto et al. 2006; Zhu et al. 1997). The effect of the RhoA kinase inhibitor, Y-27632 (1 μM; Fujimoto et al. 2006) was investigated both in the absence or presence of 4-aminopyridine (1 mM). We also investigated the effect of the gap junction inhibitor 18-α-glycyrrhetinic acid (10 μM) on capsaicin-induced relaxations. Where indicated, the endothelium was removed with a human hair, and removal was confirmed by observing a relaxation of less than 10% of precontraction of U46619 after addition of substance P.
Further, in porcine distal coronary artery, we also studied the effect of repeated administration of capsaicin (50 μM, four times) to verify the reproducibility of the responses in view of possible depletion of endogenous peptide pools or other agents.
Measurements of cAMP
Human proximal and distal, as well as porcine distal, coronary artery segments were incubated in a medium containing isobutylmethylxanthine (0.5 mM) for 30 min in the absence or presence of olcegepant (1 μM). The arterial segments were exposed to KCl (30 mM), challenged with ethanol (vehicle of capsaicin, final concentration in the baths 0.56%), capsaicin (10 μM) or h-αCGRP (100 nM, serving as a positive control) for 5 min and then snap frozen. The samples were stored at −80°C until cyclic adenosine monophosphate (cAMP) assay. To determine cAMP, tissues were homogenised in 0.5 ml 0.1 M HCl using a stainless steel ultraturrax (Polytron, Staufen, Germany). Homogenates were centrifuged at 3,300×g, and cAMP was measured in 300 μl supernatant using the ELISA kit according to the instructions of the manufacturer (R&D Systems Europe, Abingdom, UK).
Measurements of CGRP in organ bath fluid
Human distal coronary artery segments were subjected to a similar protocol as during the functional studies, while bath fluids were collected after construction of the concentration response curve. The bath fluids were collected from the segments treated with vehicle and capsaicin (100 μM), and Krebs solution was used as a control. Bath fluids were stored in tubes containing aprotinin (0.6 TIU/ml) and stored at −80°C. A competitive radioimmunoassay (Peninsula Lab INC., San Carlos, CA, USA) was used according to the instructions of the manufacturer to measure the CGRP concentrations in the bath fluid.
Data presentation and statistical analysis
The relaxant responses elicited by the agonists are expressed as percentage relaxation of the tone induced by 30 mM KCl or U46619 (in case of the K+ channel blockers). All data are presented as means±SEM, and n represents the number of blood vessel segments, all obtained from different subjects. The effect of all potential inhibitors of the relaxations to capsaicin was investigated in a paired parallel setup; that is, relaxations in segments with inhibitors were always compared to relaxations obtained in control segments from the same subject. The concentration response curves for all agonists were analysed using nonlinear regression analysis, and the potency of agonists was expressed as pEC50 using Graph Pad Prism 3.01 (Graph Pad Software, San Diego, CA, USA), setting the Emax of capsaicin in the presence of potential inhibitors to that in the respective control segment in case it was lower than the control Emax. The blocking potency of the antagonists was estimated by calculating median effective concentration (EC50) ratios, and apparent pKB values were calculated for the antagonists at each given concentration, with the slope set to unity. Statistical differences between concentration response curves to capsaicin in the absence and presence of potential inhibitors were determined using Student’s paired t-test with α set to 1.00–0.95(1/n) (Motulsky 2003) to correct for multiple comparisons. For the measurements of CGRP in the organ bath fluids, we could not exclude a non-Gaussian distribution due to the large degree of variability in the data. Therefore, the levels of CGRP in bath fluids were analysed by the non-parametric Kruskal–Wallis test, followed by Dunn’s post hoc multiple comparison test. Significance was assumed at P ≤ 0.05.
Compounds
Human α-CGRP and α-CGRP8–37 were obtained from Polypeptide, (Wolfenbüttel, Germany), olcegepant (BIBN4096BS, 1-piperidinecarboxamide, N-[2-[[5-amino-1-[[4-(4-pyridinyl)-1-piperazinyl]carbonyl] pentyl] amino]-1-[(3,5-dibromo-4-hydroxyphenyl) methyl]-2-oxoethyl]-4-(1,4-dihydro-2-oxo-3(2H)-quinazolinyl)-, [R-(R*,S*)]-) was a gift from Boehringer Ingelheim Pharma (Biberach/Riss, Germany); 4-aminopyridine was purchased from ICN Biomedicals (Aurora, OH, USA); L-733060 was purchased from Tocris (Bristol, UK); apamin, capsaicin, capsazepine, 3-isobutyl-1-methyl-xanthine, l-NAME, charybdotoxin, ruthenium red, substance P, U46619 and Y-27632 were purchased from Sigma-Aldrich (Zwijndrecht, The Netherlands), and KCl was obtained from Merck (Darmstadt, Germany). Capsaicin was dissolved in 70% ethanol, and the dilution series was also prepared in ethanol 70%. Capsazepine was dissolved in methanol; olcegepant was dissolved in a small amount of 1 N HCl and then diluted with distilled water. The other compounds were dissolved in distilled water, and all compounds were stored in aliquots at −80°C.
Results
Functional studies
Human arteries
Substance P relaxed artery segments precontracted with U46619 (10 nM–1 μM); responses were equi-efficacious in distal coronary (80 ± 5% of contraction to U46619, n = 28) and meningeal (75 ± 8%, n = 8) artery and significantly less in proximal coronary artery (27 ± 15%, n = 4). Both in the meningeal and distal coronary arteries, capsaicin induced concentration-dependent relaxations. In human proximal coronary artery, relaxant responses were only observed at the highest concentration of 100 μM, and the maximum relaxant response (34 ± 14% of contraction to 30 mM KCl) was significantly less than that observed in the distal arteries (94 ± 1% of contraction to 18–30 mM KCl). In human meningeal arteries, there was no difference in capsaicin-induced responses between arteries obtained perioperatively or postmortem; therefore, these data were pooled for further analysis. Capsaicin was equipotent and equi-efficacious in human distal coronary and human meningeal artery. In human distal coronary artery segments, the lower concentrations of capsaicin (0.1 nM–1 μM) in some cases induced contractions, but in all cases, we uniformly only measured the relaxant responses. The relaxations to capsaicin in proximal and distal coronary as well as meningeal arterial segments were insensitive to blockade by the CGRP antagonist olcegepant (1 μM; Fig. 1, Table 1).
Fig. 1.
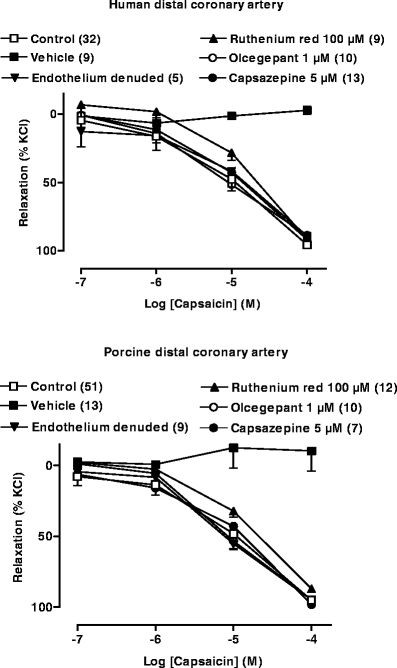
Effect of capsaicin or its vehicle in the absence or presence of various pharmacological agents or interventions in precontracted human and porcine distal coronary arteries
Table 1.
Effect of various antagonists/interventions on capsaicin-induced relaxations in human isolated artery segments
| Antagonist or other intervention (n) | Emax (%) | Δ Emax | pEC50 | Δ pEC50 |
|---|---|---|---|---|
| Human distal coronary artery | ||||
| (Control) (32) | 94 ± 1 | 5.27 ± 0.12 | ||
| Olcegepant (1 μM) (10) | 89 ± 4 | 8 ± 4 | 4.84 ± 0.09 | 0.33 ± 0.16 |
| CGRP8–37 (10 μM) (5) | 96 ± 3 | 3 ± 4 | 4.79 ± 0.06 | 0.16 ± 0.08 |
| Capsazepine (5 μM) (13) | 91 ± 3 | 1 ± 2 | 5.10 ± 0.13 | 0.07 ± 0.16 |
| Ruthenium red (0.1 mM) (9) | 92 ± 3 | 5 ± 3 | 5.01 ± 0.13 | 0.21 ± 0.22 |
| L-733060 (5 μM) (7) | 94 ± 2 | 2 ± 2 | 6.03 ± 0.78 | −0.54 ± 0.34 |
| Denuded endothelium (5) | 90 ± 6 | 9 ± 5 | 5.34 ± 0.48 | −0.21 ± 0.58 |
| l-NAME (0.1 mM) (7) | 94 ± 2 | 3 ± 2 | 5.23 ± 0.48 | −0.24 ± 0.45 |
| 18-α-Glycyrrhetinic acid (10 μM) (3) | 96 ± 2 | 0 ± 2 | 5.08 ± 0.28 | −0.15 ± 0.38 |
| Olcegepant (1 μM) + L-733060 (5 μM) (3) | 90 ± 6 | 1 ± 3 | 6.04 ± 0.23 | 0.06 ± 0.21 |
| (Control) (10) | 97 ± 1 | 5.91 ± 0.32 | ||
| 4-Aminopyridine (1 mM) (6) | 95 ± 2 | 2 ± 3 | 5.64 ± 0.38 | 0.94 ± 0.46 |
| Charybdotoxin (0.5 μM) + apamin (0.1 μM) (8) | 97 ± 1 | 0 ± 1 | 5.80 ± 0.37 | 0.23 ± 0.37 |
| Iberiotoxin (0.5 μM) + apamin (0.1 μM) (5) | 96 ± 2 | 0 ± 2 | 5.94 ± 0.65 | 0.15 ± 0.38 |
| Y-276323 (1 μM) (3) | 99 ± 1 | −1 ± 2 | 5.39 ± 0.21 | −0.58 ± 0.14 |
| Y-276323 (1 μM) + 4-Aminopyridine (1 mM) (3) | 100 ± 0 | −3 ± 4 | 5.12 ± 0.18 | 0.33 ± 0.28 |
| Human proximal coronary artery | ||||
| (Control) (4) | 34 ± 14 | 4.30 ± 0.14 | ||
| Olcegepant (1 μM) (4) | 36 ± 16 | 4.40 ± 0.17 | ||
| Human meningeal artery | ||||
| (Control) (10) | 91 ± 5 | 5.04 ± 0.09 | ||
| Olcegepant (1 μM) (10) | 96 ± 1 | −5 ± 4 | 5.03 ± 0.07 | −0.07 ± 0.08 |
| Capsazepine (5 μM) (4) | 81 ± 9 | 8 ± 7 | 4.90 ± 0.31 | 0.11 ± 0.31 |
| Ruthenium red (0.1 mM) (3) | 74 ± 15 | 5 ± 14 | 5.13 ± 0.42 | 0.02 ± 0.62 |
| L-733060 (5 μM) (4) | 79 ± 12 | 8 ± 15 | 4.80 ± 0.31 | −0.09 ± 0.31 |
Emax is the maximum relaxant response, expressed as percentage of the respective precontraction; pEC50 is the −logEC50, where EC50 is the concentration of agonist required to produce half the maximal response. The arteries were precontracted with KCl (18–30 mM) except where KCl (45–90 mM; bold) or U46619 (ital).
As the relaxant responses to capsaicin were small in human proximal coronary arteries and the availability of human meningeal arteries was very limited, further experiments were only carried out in human distal coronary artery. In this preparation, the CGRP receptor antagonist CGRP8–37 (10 μM), the TRPV1 receptor antagonist capsazepine (5 μM) and the NK1 receptor antagonist L-733006 (0.5 μM) also did not attenuate capsaicin-induced relaxations (Table 1). Similarly, there was no significant difference in relaxant responses in endothelium-intact or endothelium-denuded segments (Table 1). Also, in the absence or presence of the NO synthase inhibitor l-NAME (100 μM) or in the presence of the gap junction blocker 18-α-glycyrrhetinic acid, capsaicin caused equipotent relaxations compared to the respective control segments (Table 1). Various K+ channel blockers, namely, 4-aminopyridine (1 mM), charybdotoxin (0.5 μM) + apamin (0.1 μM) and iberiotoxin (0.1 μM) + apamin (0.1 μM) were also unable to block the relaxant responses to capsaicin. The RhoA kinase inhibitor Y-27632 (1 μM) alone or in combination with 4-aminopyridine was also unable to block the relaxant responses to capsaicin. Ruthenium red (100 μM), a nonselective blocker of Ca2+ transport through membrane channels, also did not significantly block these responses (Table 1). It is noteworthy that at higher concentrations, capsazepine and L-733060 significantly attenuated the responses to their respective preconstriction agents, and also in case of preincubation with Y-27632 and ruthenium red, 2- to 20-fold higher concentrations of precontracting agents were required to induce 60–75% of the maximal contraction reached with KCl (100 mM). At still higher concentrations of capsazepine (>5 μM) and L-733060 (>0.5 μM), even higher concentrations of preconstricting agents could not produce a workable precontraction. Additionally, the combination of olcegepant (1 μM) + L-733060 (0.5 μM) in human distal coronary artery or 4-aminopyridine (1 mM) + Y-27632 (1 μM) both in human and porcine distal coronary artery were also unable to block the responses to capsaicin. The vehicle of capsaicin (0.56% ethanol) did not induce any significant relaxations. It should be noted that the potency of capsaicin was higher on a precontraction with U46619 (10 nM–1 μM; pEC50 of capsaicin, 5.91 ± 0.32) than on a precontraction with KCl (18–30 mM; pEC50 of capsaicin, 5.27 ± 0.12; P = 0.029). However, irrespective of the precontracting agent used, the antagonists behaved in a similar fashion towards capsaicin. For example, the effects of 4-aminopyridine on capsaicin-induced relaxations were similar after precontraction with KCl (pEC50, 5.01 ± 0.05 and 5.12 ± 0.41; n = 7, in the absence and presence of 4-aminopyridine, respectively) and with U46619 (pEC50, 6.58 ± 0.47 and 5.64 ± 0.38; n = 6, in the absence and presence of 4-aminopyridine, respectively). In human meningeal artery, capsazepine, L-733006 and also ruthenium red did not block the responses to capsaicin (Table 1).
Porcine arteries
In porcine arteries, substance P induced relaxations in precontracted arteries to a varying degree in different vessels. The relaxations in distal coronary (64 ± 6% of contraction to U46619, 10 nM–1 μM, n = 26) basilar (44 ± 3%, n = 3) and meningeal (57 ± 15%, n = 3) artery were similar, whereas that in proximal coronary (8 ± 8%, n = 4) artery was significantly smaller. In porcine proximal and distal coronary arteries, capsaicin induced concentration-dependent relaxations. Unlike in human coronary arteries, the maximum response was not significantly different between porcine proximal and distal coronary arteries (Table 2). In both the arteries, olcegepant (1 μM) did not block the responses to capsaicin. Further experiments were carried out in distal coronary arteries and, similar as in the human distal coronary artery, capsazepine (1 μM), ruthenium red (0.1 mM), L-73360 (0.5 μM), 4-aminopyridine (1 mM), l-NAME (0.1 mM), charybdotoxin (0.5 μM) + apamin (0.1 μM), iberiotoxin (0.5 μM) + apamin (0.1 μM), Y-27632 (1 μM), 18-α-glycyrrhetinic acid (10 μM) and endothelium denudation did not affect capsaicin-induced relaxations (Fig. 1, Table 2). Additionally, Y-27632 (1 μM), alone or combined with 4-aminopyridine (1 mM), was also unable to block the responses to capsaicin. Similar as in the other arteries, the vehicle did not induce a relaxation (Fig. 1). Interestingly, in contrast to what we observed in the human distal coronary artery, precontracting the arteries with either KCl or U46619 did not change the relaxant responses to capsaicin. Four consecutive challenges to capsaicin (50 μM) did not significantly affect the magnitude of the responses (Fig. 2).
Table 2.
Effect of various antagonists/interventions on capsaicin-induced relaxations in porcine isolated artery segments
| Antagonist or other intervention (n) | Emax (%) | Δ Emax | pEC50 | Δ pEC50 |
|---|---|---|---|---|
| Porcine distal coronary artery | ||||
| (Control) (56) | 96 ± 1 | 5.27 ± 0.09 | ||
| Olcegepant (1 μM) (10) | 92 ± 2 | 3 ± 2 | 5.26 ± 0.11 | −0.02 ± 0.11 |
| CGRP8–37 (10 μM) (5) | 97 ± 3 | −2 ± 5 | 4.78 ± 0.06 | 0.00 ± 0.03 |
| Capsazepine (5 μM) (7) | 99 ± 1 | −3 ± 2 | 5.15 ± 0.26 | 0.26 ± 0.16 |
| Ruthenium red (0.1 mm) (12) | 88 ± 3 | 7 ± 4 | 4.89 ± 0.09 | 0.47 ± 0.23 |
| L-733060 (5 μM) (7) | 88 ± 3 | 4 ± 4 | 4.89 ± 0.09 | −0.04 ± 0.15 |
| Denuded endothelium (9) | 91 ± 4 | 2 ± 2 | 5.62 ± 0.49 | 0.21 ± 0.16 |
| l-NAME (0.1 mM) (6) | 90 ± 8 | 8 ± 8 | 4.84 ± 0.15 | 0.28 ± 0.31 |
| (Control) (15) | 99 ± 0 | 5.08 ± 0.15 | ||
| 18-α-Glycyrrhetinic acid (10 μM) (3) | 99 ± 1 | 0 ± 1 | 5.73 ± 0.75 | −0.59 ± 0.53 |
| 4-Aminopyridine (1 mM) (7) | 96 ± 2 | 3 ± 2 | 4.84 ± 0.15 | −0.46 ± 0.35 |
| Charybdotoxin (0.5 μM) + apamin (0.1 μM) (11) | 99 ± 1 | 0 ± 1 | 5.27 ± 0.15 | −0.04 ± 0.20 |
| Y-276323 (1 μM) (9) | 96 ± 2 | 3 ± 2 | 5.17 ± 0.19 | 0.04 ± 0.21 |
| Y-276323 (1 μM) + 4-Aminopyridine (1 mM) (8) | 96 ± 3 | 4 ± 3 | 5.22 ± 0.20 | 0.00 ± 0.11 |
| Porcine proximal coronary artery | ||||
| (Control) (4) | 100 ± 0 | 5.33 ± 0.42 | ||
| Olcegepant (1 μM) (4) | 90 ± 6 | 5.79 ± 0.16 | ||
| Porcine basilar artery | ||||
| (Control) (3) | 97 ± 1 | 4.70 ± 0.05 | ||
| Olcegepant (1 μM) (3) | 100 ± 0 | 4.97 ± 0.24 | ||
| Capsazepine (5 μM) (3) | 100 ± 0 | 4.80 ± 0.01 | ||
| Porcine meningeal artery | ||||
| (Control) (3) | 99 ± 1 | 4.82 ± 0.02 | ||
| Olcegepant 1 μM (3) | 99 ± 1 | 4.88 ± 0.04 | ||
Emax is the maximum relaxant response, expressed as percentage of the respective precontraction; pEC50 is the −logEC50, where EC50 is the concentration of agonist required to produce half the maximal response. The arteries were precontracted with KCl (18–30 mM) except where KCl (45–90 mM; bold) or U46619 (ital).
Fig. 2.
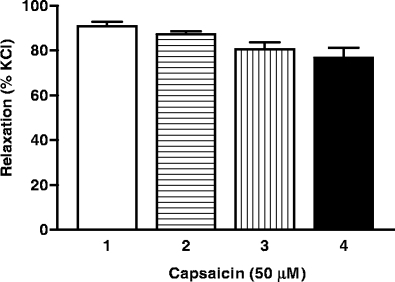
Effect of four consecutive challenges to capsaicin (50 μM) in porcine distal coronary arteries precontracted with KCl (30 mM)
There were no significant differences in efficacy or potency of capsaicin in porcine basilar (Emax, 97 ± 1%; pEC50, 4.70 ± 0.05; n = 3) and meningeal (Emax, 99 ± 1%; pEC50, 4.82 ± 0.02; n = 3) arteries as compared to porcine distal coronary arteries. In both these arteries, responses to capsaicin were not affected by olcegepant (1 μM).
Measurements of cAMP
Capsaicin (10 μM) did not affect cAMP levels in comparison to its vehicle or the control, which was only exposed to 30 mM KCl. In contrast, αCGRP (100 nM), which was used as a positive control, significantly increased cAMP levels in both human and porcine distal coronary arteries, which was blocked after incubation with olcegepant (1 μM; Fig. 3).
Fig. 3.
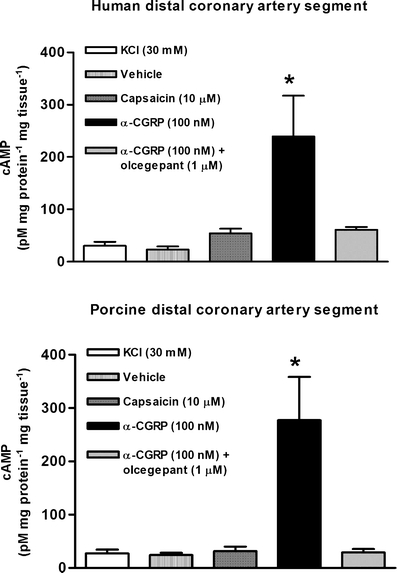
Changes in cAMP levels in human (n = 4–7) and porcine (n = 4–9) distal coronary artery segments after exposure to various pharmacological agents. *Significantly different (P < 0.05) from KCl (30 mM)-treated segments
Measurements of CGRP levels in organ bath fluid
Capsaicin and its vehicle induced a significant CGRP release from human distal coronary artery segments into the organ bath fluid (Fig. 4) as compared to levels observed in Krebs solution. There was no significant difference between CGRP levels obtained after incubation with capsaicin or its vehicle.
Fig. 4.
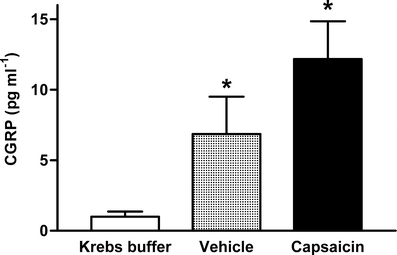
CGRP levels measured in bath fluids (Krebs buffer, control) after capsaicin or vehicle challenge in precontracted human distal coronary artery segments. *Significantly different (P < 0.05) from Krebs buffer
Discussion
In the present study, we investigated the role of CGRP in capsaicin-induced relaxations in human and porcine isolated arteries. In all arteries investigated, there does not seem to be any relevant role of CGRP in the relaxant responses to capsaicin. Further, the effects of capsaicin appear to be mediated by non-specific mechanisms.
In all arteries investigated, capsaicin induced concentration-dependent relaxations, although these had a limited potency and efficacy in human proximal coronary artery. The responses to capsaicin were resistant to blockade with olcegepant in all tissues studied, which suggests no involvement of CGRP receptors. The potency of capsaicin is in line with what has earlier been observed in human coronary arteries (Franco-Cereceda 1991a) and guinea pig ileum (Fujimoto et al. 2006). As expected in view of its resistance to olcegepant, CGRP8–37 (10 μM) also did not block capsaicin-induced relaxations in human distal coronary artery segments. This is contrary to the observations by Franco-Cereceda and Rudehill (1989), where the authors claim the relaxations to capsaicin are mediated by CGRP on the basis of the observation that the sustained relaxations in the human coronary induced by capsaicin are similar to those induced by exogenous CGRP, unlike the transient relaxations to substance P, which are followed by rapid tachyphylaxis. In another publication, these authors claim involvement of CGRP in responses to capsaicin in the human isolated coronary artery on the basis of increased CGRP-like immunoreactivity after exposure of the artery segments to capsaicin (Franco-Cereceda 1991b). It should be noted that in the above study, the authors show an increase in CGRP-like immunoreactivity in large arteries, where no functional studies were performed. Further, the classical prerequisite for demonstrating the involvement of a particular pharmacological agent, by using the corresponding antagonist to block functional responses, was lacking in this study. Admittedly, the same group did demonstrate that CGRP8–37 inhibited the responses to capsaicin in porcine coronary arteries (Franco-Cereceda 1991b), although we could not block these responses even at 10-μM concentration.
As CGRP-induced responses are mediated by increases in cAMP (Gupta et al. 2006c), we measured the levels of this second messenger after exposure to capsaicin and CGRP. We observed no increase in cAMP after addition of capsaicin, in contrast to the increased levels of cAMP after exposure of the vessel segments to (exogenous) α-CGRP. These increased levels were, as expected, blocked by the CGRP receptor antagonist olcegepant. Interestingly, after exposure to capsaicin, we observed CGRP release in the organ bath fluid, where the human distal coronary arteries were mounted in accordance with earlier observations (Franco-Cereceda 1991a). However, a similar increase was observed after administration of vehicle, while the control was not studied in the study of Franco-Cereceda et al. (1991a). Although capsaicin is known to activate TRPV1, there are reports that ethanol, also via activation of TRPV1, induces the release of CGRP as well (Trevisani et al. 2002, 2004). Moreover, as obvious from Fig. 1, the vehicle did not induce any relaxations in the precontracted arteries, and hence, the released CGRP cannot account for relaxations induced by capsaicin. Interestingly, the concentration of CGRP detected in the bath fluid of about 3 pM should have been about 10,000 times higher in the vessel segments (∼0.5 mg tissue in 5-ml organ bath fluid). Thus, the concentration of CGRP in the vessel segments should have been in the nanomolar range, which is equal to or even higher than the pEC50 in human distal coronary artery under similar experimental conditions (Gupta et al. 2006c) and should, thus, have induced a detectable relaxation. Therefore, it is most likely that the radioimmunoassay displayed cross-reactivity to another ligand, not CGRP. Admittedly, our observation that ruthenium red, even at a very high concentration (100 μM), did not block the responses to capsaicin, is in contrast with the observations described by Franco-Cereceda (1991a), where ruthenium red completely blocked the responses to capsaicin. Additional evidence for the fact that CGRP and TRPV1 are not involved in relaxant responses to capsaicin is provided by the fact that capsazepine, a competitive antagonist of TRPV1 (Caterina et al. 1997), did not block responses to capsaicin in our study.
In view of reports of involvement of various K+ channels in the relaxant responses to capsaicin in various smooth muscle preparations (Yeon et al. 2001; Zhu et al. 1997), we also investigated various K+ channel inhibitors in similar or even higher concentrations, but none of these blocked the responses to capsaicin. 4-Aminopyridine, a blocker of delayed rectifier K+ channels, which is reported to block the responses to capsaicin in rabbit coronary artery (Yeon et al. 2001) and guinea pig ileum (Fujimoto et al. 2006), was ineffective in human as well as porcine distal coronary artery. Similarly, charybdotoxin, a BKCa and IKCa blocker, which blocked capsaicin responses in equine tracheal smooth muscle (Zhu et al. 1997), did not block these responses at similar concentrations and even in combination with apamin, a SKCa blocker. We also used the RhoA kinase inhibitor Y-27632, but unlike in guinea pig ileum, (Fujimoto et al. 2006) it also did not antagonise responses to capsaicin in the isolated preparations used in the present study. The capsaicin-mediated responses appear to be endothelium-independent, as denudation of the endothelium did not significantly change the capsaicin-induced responses. Further, the vehicle of capsaicin was without effect and, hence, cannot account for the relaxant responses to capsaicin. Moreover, repeated administration of capsaicin in porcine coronary artery did not significantly decrease the responses, suggesting that stored neuropeptides are not responsible for the relaxations, as these would most likely be depleted after repeated challenges to capsaicin (Sams-Nielsen et al. 2001). Unlike in the present study, relaxations in precontracted guinea pig isolated pulmonary artery could not be repeated with capsaicin indicating depletion of neurotransmitters (Maggi et al. 1990). Although we did not include a positive control for the various K+ blockers in the current study, in the same set up at our laboratory, relaxant responses to l-S-nitrosocysteine were blocked by the combination of charybdotoxin (100 nM) and apamin (100 nM) in porcine distal arteries (Batenburg et al. 2004b). Further, the concentrations of the various inhibitors that we used in our study were equal to (Yeon et al. 2001; Zhu et al. 1997) or even higher (Yeon et al. 2001) than those employed by others.
Taken together, our observations in human and porcine distal coronary artery suggest that capsaicin-induced responses are not mediated by CGRP, substance P or TRPV1 receptors and also do not involve various Ca2+-activated K+ channels. The relaxations to capsaicin are mediated by a cAMP independent pathway. The major component of capsaicin-induced relaxations, therefore, appears to be mediated by non-specific actions of capsaicin, rather than from the release of neuropeptides like CGRP.
References
- Akerman S, Kaube H, Goadsby PJ (2003) Vanilloid type 1 receptors (VR1) on trigeminal sensory nerve fibres play a minor role in neurogenic dural vasodilatation, and are involved in capsaicin-induced dural dilation. Br J Pharmacol 140:718–724 [DOI] [PMC free article] [PubMed]
- Arulmani U, Heiligers JPC, Garrelds IM, Sánchez-López A, Willems EW, Villalón CM, Saxena PR (2004a) Effects of sumatriptan on capsaicin-induced carotid haemodynamic changes and CGRP release in anaesthetized pigs. Cephalalgia 24:717–727 [DOI] [PubMed]
- Arulmani U, MaassenVanDenBrink A, Villalón CM, Saxena PR (2004b) Calcitonin gene-related peptide and its role in migraine pathophysiology. Eur J Pharmacol 500:315–330 [DOI] [PubMed]
- Arulmani U, Gupta S, MaassenVanDenBrink A, Centurión D, Villalón CM, Saxena PR (2006) Experimental migraine models and their relevance in migraine therapy. Cephalalgia 26:642–659 [DOI] [PubMed]
- Batenburg WW, Garrelds IM, Bernasconi CC, Juillerat-Jeanneret L, van Kats JP, Saxena PR, Danser AHJ (2004a) Angiotensin II type 2 receptor-mediated vasodilation in human coronary microarteries. Circulation 109:2296–2301 [DOI] [PubMed]
- Batenburg WW, Popp R, Fleming I, de Vries R, Garrelds IM, Saxena PR, Danser AHJ (2004b) Bradykinin-induced relaxation of coronary microarteries: S-nitrosothiols as EDHF? Br J Pharmacol 142:125–135 [DOI] [PMC free article] [PubMed]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389:816–824 [DOI] [PubMed]
- Chai W, Mehrotra S, Danser AHJ, Schoemaker RG (2006) The role of calcitonin gene-related peptide (CGRP) in ischemic preconditioning in isolated rat hearts. Eur J Pharmacology 531:246–253 [DOI] [PubMed]
- Delepine L, Aubineau P (1997) Plasma protein extravasation induced in the rat dura mater by stimulation of the parasympathetic sphenopalatine ganglion. Exp Neurol 147:389–400 [DOI] [PubMed]
- Dong YL, Green KE, Vegiragu S, Hankins GD, Martin E, Chauhan M, Thota C, Yallampalli C (2005) Evidence for decreased calcitonin gene-related peptide (CGRP) receptors and compromised responsiveness to CGRP of fetoplacental vessels in preeclamptic pregnancies. J Clin Endocrinol Metab 90:2336–2343 [DOI] [PubMed]
- Doods H, Hallermayer G, Wu D, Entzeroth M, Rudolf K, Engel W, Eberlein W (2000) Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br J Pharmacol 129:420–423 [DOI] [PMC free article] [PubMed]
- Edvinsson L, Ekman R, Jansen I, McCulloch J, Uddman R (1987) Calcitonin gene-related peptide and cerebral blood vessels: distribution and vasomotor effects. J Cereb Blood Flow Metab 7:720–728 [DOI] [PubMed]
- Edvinsson L, Alm R, Shaw D, Rutledge RZ, Koblan KS, Longmore J, Kane SA (2002) Effect of the CGRP receptor antagonist BIBN4096BS in human cerebral, coronary and omental arteries and in SK-N-MC cells. Eur J Pharmacol 434:49–53 [DOI] [PubMed]
- Franco-Cereceda A (1988) Calcitonin gene-related peptide and tachykinins in relation to local sensory control of cardiac contractility and coronary vascular tone. Acta Physiol Scand Suppl 569:1–63 [PubMed]
- Franco-Cereceda A (1991a) Calcitonin gene-related peptide and human epicardial coronary arteries: presence, release and vasodilator effects. Br J Pharmacol 102:506–510 [DOI] [PMC free article] [PubMed]
- Franco-Cereceda A (1991b) Resiniferatoxin-, capsaicin- and CGRP-evoked porcine coronary vasodilatation is independent of EDRF mechanisms but antagonized by CGRP(8–37). Acta Physiol Scand 143:331–337 [DOI] [PubMed]
- Franco-Cereceda A, Rudehill A (1989) Capsaicin-induced vasodilatation of human coronary arteries in vitro is mediated by calcitonin gene-related peptide rather than substance P or neurokinin A. Acta Physiol Scand 136:575–580 [DOI] [PubMed]
- Franco-Cereceda A, Rudehill A, Lundberg JM (1987) Calcitonin gene-related peptide but not substance P mimics capsaicin-induced coronary vasodilation in the pig. Eur J Pharmacol 142:235–243 [DOI] [PubMed]
- Franco-Cereceda A, Lundberg JM, Saria A, Schreibmayer W, Tritthart HA (1988) Calcitonin gene-related peptide: release by capsaicin and prolongation of the action potential in the guinea-pig heart. Acta Physiol Scand 132:181–190 [DOI] [PubMed]
- Fujimoto S, Mori M, Tsushima H, Kunimatsu M (2006) Capsaicin-induced, capsazepine-insensitive relaxation of the guinea-pig ileum. Eur J Pharmacol 530:144–151 [DOI] [PubMed]
- Gazzieri D, Trevisani M, Tarantini F, Bechi P, Masotti G, Gensini GF, Castellani S, Marchionni N, Geppetti P, Harrison S (2006) Ethanol dilates coronary arteries and increases coronary flow via transient receptor potential vanilloid 1 and calcitonin gene-related peptide. Cardiovasc Res 70:589–599 [DOI] [PubMed]
- Goadsby PJ, Lipton RB, Ferrari MD (2002) Migraine—current understanding and treatment. N Engl J Med 346:257–270 [DOI] [PubMed]
- Gunthorpe MJ, Benham CD, Randall A, Davis JB (2002) The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci 23:183–191 [DOI] [PubMed]
- Gupta S, Akerman S, van den Maagdenberg AMJM, Saxena PR, Goadsby PJ, MaassenVanDenBrink A (2006a) Intravital microscopy on a closed cranial window in mice: a model to study trigeminovascular mechanisms involved in migraine. Cephalalgia 26(11):1294–1303 [DOI] [PubMed]
- Gupta S, Mehrotra S, Avezaat CJJ, Villalón CM, Saxena PR, MaassenVanDenBrink A (2006b) Characterisation of CGRP receptors in the human isolated middle meningeal artery. Life Sci 79:265–271 [DOI] [PubMed]
- Gupta S, Mehrotra S, Villalón CM, Garrelds IM, de Vries R, van Kats JP, Sharma HS, Saxena PR, MaassenVanDenBrink A (2006c) Characterisation of CGRP receptors in human and porcine isolated coronary arteries: evidence for CGRP receptor heterogeneity. Eur J Pharmacol 530:107–116 [DOI] [PubMed]
- Maggi CA, Patacchini R, Perretti F, Tramontana M, Manzini S, Geppetti P, Santicioli P (1990) Sensory nerves, vascular endothelium and neurogenic relaxation of the guinea-pig isolated pulmonary artery. Naunyn Schmiedebergs Arch Pharmacol 342:78–84 [DOI] [PubMed]
- Mitchell RW, Ndukwu IM, Herrnreiter A, Uzendoski K, Gitter B, Solway J, Leff AR (1995) Differential tachykinin receptor subtype activation in capsaicin and KCl contractions of guinea pig trachealis. Am J Physiol 269:L837–L842 [DOI] [PubMed]
- Motulsky HJ (2003) Prism 4 statistics guide—statistical analyses for laboratory and clinical researchers. GraphPad Software Inc., San Diego, CA. www.graphpad.com
- Nyborg NC, Baandrup U, Mikkelsen EO, Mulvany MJ (1987) Active, passive and myogenic characteristics of isolated rat intramural coronary resistance arteries. Pflügers Arch 410:664–670 [DOI] [PubMed]
- Olesen J, Diener HC, Husstedt IW, Goadsby PJ, Hall D, Meier U, Pollentier S, Lesko LM (2004) Calcitonin gene-related peptide receptor antagonist BIBN4096BS for the acute treatment of migraine. N Engl J Med 350:1104–1110 [DOI] [PubMed]
- Sams-Nielsen A, Orskov C, Jansen-Olesen I (2001) Pharmacological evidence for CGRP uptake into perivascular capsaicin sensitive nerve terminals. Br J Pharmacol 132:1145–1153 [DOI] [PMC free article] [PubMed]
- Seabrook GR, Shepheard SL, Williamson DJ, Tyrer P, Rigby M, Cascieri MA, Harrison T, Hargreaves RJ, Hill RG (1996) L-733,060, a novel tachykinin NK1 receptor antagonist; effects in [Ca2+]$$_{{\text{i}}}$$ mobilisation, cardiovascular and dural extravasation assays. Eur J Pharmacol 317:129–135 [DOI] [PubMed]
- Szallasi A (2006) Small molecule vanilloid TRPV1 receptor antagonists approaching drug status: can they live up to the expectations? Naunyn Schmiedebergs Arch Pharmacol 373:273–286 [DOI] [PubMed]
- Tang YH, Lu R, Li YJ, Deng HW, Liu GZ (1997) Protection by capsaicin against attenuated endothelium-dependent vasorelaxation due to lysophosphatidylcholine. Naunyn Schmiedebergs Arch Pharmacol 356:364–367 [DOI] [PubMed]
- Tang ZL, Dai W, Li YJ, Deng HW (1999) Involvement of capsaicin-sensitive sensory nerves in early and delayed cardioprotection induced by a brief ischaemia of the small intestine. Naunyn Schmiedebergs Arch Pharmacol 359:243–247 [DOI] [PubMed]
- Trevisani M, Gazzieri D, Benvenuti F, Campi B, Dinh QT, Groneberg DA, Rigoni M, Emonds-Alt X, Creminon C, Fischer A, Geppetti P, Harrison S (2004) Ethanol causes inflammation in the airways by a neurogenic and TRPV1-dependent mechanism. J Pharmacol Exp Ther 309:1167–1173 [DOI] [PubMed]
- Trevisani M, Smart D, Gunthorpe MJ, Tognetto M, Barbieri M, Campi B, Amadesi S, Gray J, Jerman JC, Brough SJ, Owen D, Smith GD, Randall AD, Harrison S, Bianchi A, Davis JB, Geppetti P (2002) Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci 5:546–551 [DOI] [PubMed]
- Yeon D, Kwon S, Lee Y, Leem J, Nam T, Ahn D (2001) Capsaicin-induced relaxation in rabbit coronary artery. J Vet Med Sci 63:499–503 [DOI] [PubMed]
- Zhou ZH, Peng J, Ye F, Li NS, Deng HW, Li YJ (2002) Delayed cardioprotection induced by nitroglycerin is mediated by alpha-calcitonin gene-related peptide. Naunyn Schmiedebergs Arch Pharmacol 365:253–259 [DOI] [PubMed]
- Zhu FX, Zhang XY, Olszewski MA, Robinson NE (1997) Mechanism of capsaicin-induced relaxation in equine tracheal smooth muscle. Am J Physiol 273:L997–L1001 [DOI] [PubMed]


