Abstract
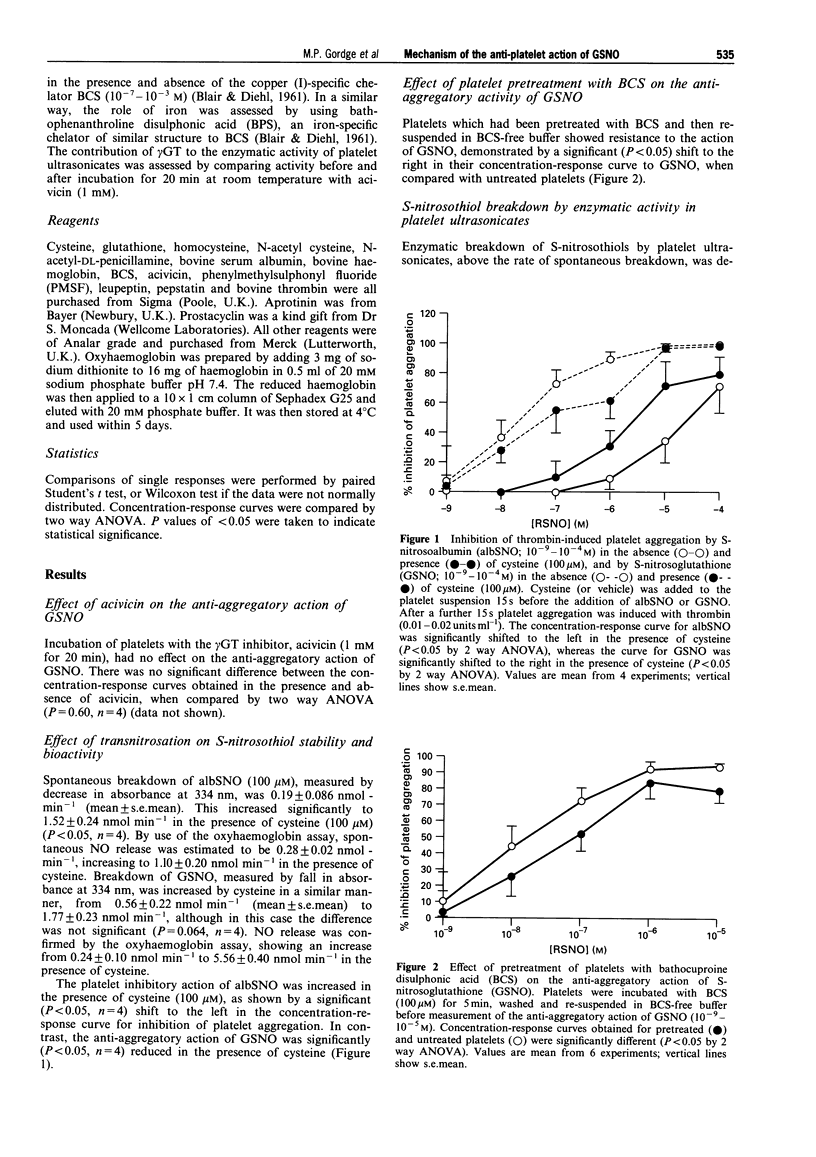
1. S-nitrosoglutathione (GSNO) is a potent and selective anti-platelet agent, despite the fact that its spontaneous rate of release of nitric oxide (NO) is very slow. Our aim was to investigate the mechanism of the anti-aggregatory action of GSNO. 2. The biological action of GSNO could be mediated by NO released from S-nitrosocystylglycine, following enzymatic cleavage of GSNO by gamma-glutamyl transpeptidase. The anti-aggregatory potency of GSNO was not, however, altered by treatment of target platelets with the gamma-glutamyl transpeptidase inhibitor acivicin (1 mM). gamma-Glutamyl transpeptidase is not, therefore, involved in mediating the action of GSNO. 3. The rate of breakdown of S-nitrosoalbumin was increased from 0.19 +/- 0.086 nmol min-1 to 1.52 +/- 0.24 nmol min-1 (mean +/- s.e.mean) in the presence of cysteine (P < 0.05, n = 4). Inhibition of platelet aggregation by S-nitrosoalbumin was also significantly increased by cysteine (P < 0.05, n = 4), suggesting that the biological activity of S-nitrosoalbumin is mediated by exchange of NO from the protein carrier to form the unstable compound cysNO. Breakdown of GSNO showed a non-significant acceleration in the presence of cysteine, from 0.56 +/- 0.22 to 1.77 +/- 0.27 nmol min-1 (mean +/- s.e.mean) (P = 0.064, n = 4), and its ability to inhibit platelet aggregation was not enhanced by cysteine. This indicates that the anti-platelet action of GSNO is not dependent upon transnitrosation to form cysNO. 4. Platelets pretreated with the copper (I)-specific chelator bathocuproine disulphonic acid (BCS), then resuspended in BCS-free buffer, showed resistance to the inhibitory effect of GSNO. These findings suggest that BCS impedes the action of GSNO by binding to structures on the platelet, rather than by chelating free copper in solution. 5. Release of NO from GSNO was catalysed enzymatically by ultrasonicated platelet suspensions. This enzyme had an apparent K(m) for GSNO of 12.4 +/- 2.64 microM and a Vmax of 0.21 +/- 0.03 nmol min-1 per 10(8) platelets (mean +/- s.e.mean, n = 5). It was inhibited by BCS, but not by the iron chelator bathophenathroline disulphonic acid, nor by acivicin. 6. We conclude that the stable S-nitrosothiol compound GSNO may exert its anti-platelet action via enzymatic, rather than spontaneous release of NO. This is mediated by a copper-dependent mechanism. The potency and platelet-selectivity of GSNO may result from targeted NO release at the platelet surface.
Full text
PDF

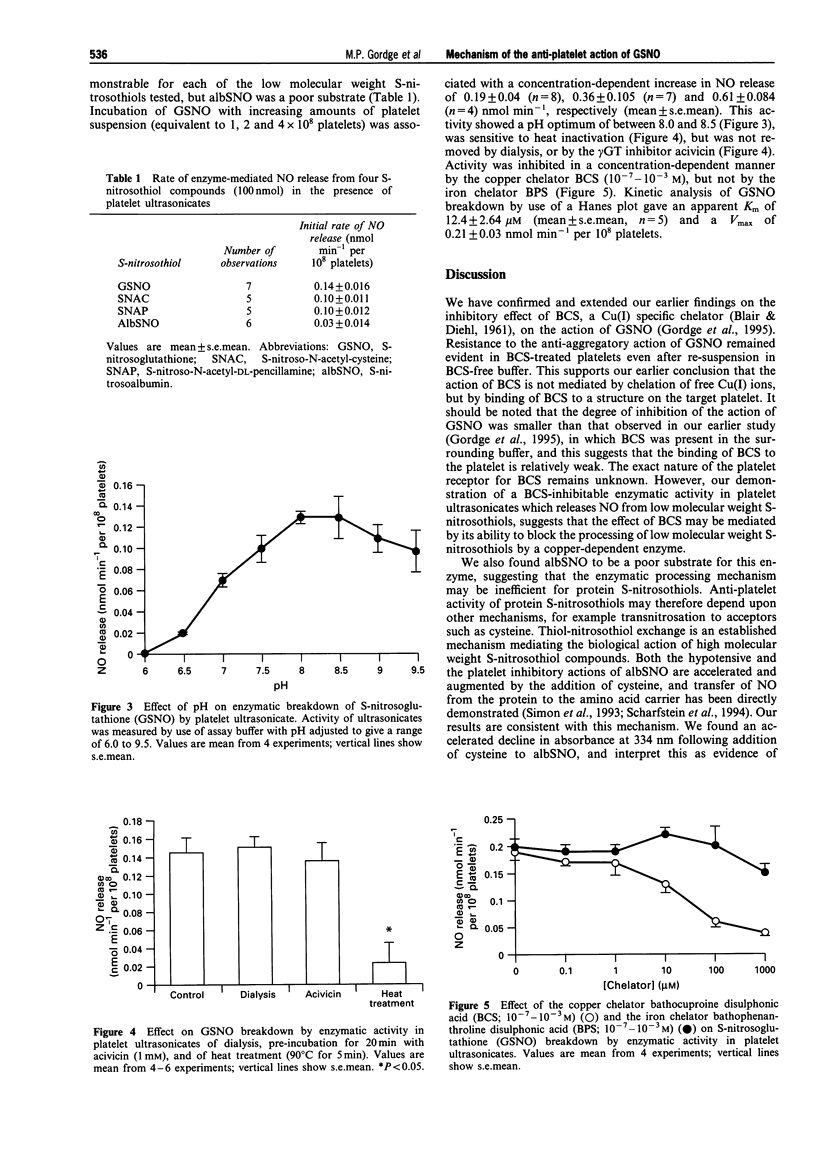


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnelle D. R., Stamler J. S. NO+, NO, and NO- donation by S-nitrosothiols: implications for regulation of physiological functions by S-nitrosylation and acceleration of disulfide formation. Arch Biochem Biophys. 1995 Apr 20;318(2):279–285. doi: 10.1006/abbi.1995.1231. [DOI] [PubMed] [Google Scholar]
- Askew S. C., Butler A. R., Flitney F. W., Kemp G. D., Megson I. L. Chemical mechanisms underlying the vasodilator and platelet anti-aggregating properties of S-nitroso-N-acetyl-DL-penicillamine and S-nitrosoglutathione. Bioorg Med Chem. 1995 Jan;3(1):1–9. doi: 10.1016/0968-0896(94)00139-t. [DOI] [PubMed] [Google Scholar]
- Clancy R. M., Abramson S. B. Novel synthesis of S-nitrosoglutathione and degradation by human neutrophils. Anal Biochem. 1992 Aug 1;204(2):365–371. doi: 10.1016/0003-2697(92)90253-4. [DOI] [PubMed] [Google Scholar]
- DOWD J. E., RIGGS D. S. A COMPARISON OF ESTIMATES OF MICHAELIS-MENTEN KINETIC CONSTANTS FROM VARIOUS LINEAR TRANSFORMATIONS. J Biol Chem. 1965 Feb;240:863–869. [PubMed] [Google Scholar]
- Feelisch M., te Poel M., Zamora R., Deussen A., Moncada S. Understanding the controversy over the identity of EDRF. Nature. 1994 Mar 3;368(6466):62–65. doi: 10.1038/368062a0. [DOI] [PubMed] [Google Scholar]
- Freedman J. E., Frei B., Welch G. N., Loscalzo J. Glutathione peroxidase potentiates the inhibition of platelet function by S-nitrosothiols. J Clin Invest. 1995 Jul;96(1):394–400. doi: 10.1172/JCI118047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston B., Drazen J. M., Jansen A., Sugarbaker D. A., Loscalzo J., Richards W., Stamler J. S. Relaxation of human bronchial smooth muscle by S-nitrosothiols in vitro. J Pharmacol Exp Ther. 1994 Feb;268(2):978–984. [PubMed] [Google Scholar]
- Gaston B., Reilly J., Drazen J. M., Fackler J., Ramdev P., Arnelle D., Mullins M. E., Sugarbaker D. J., Chee C., Singel D. J. Endogenous nitrogen oxides and bronchodilator S-nitrosothiols in human airways. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10957–10961. doi: 10.1073/pnas.90.23.10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Babbedge R., Brave S. R., Hart S. L., Hobbs A. J., Tucker J. F., Wallace P., Moore P. K. An investigation of some S-nitrosothiols, and of hydroxy-arginine, on the mouse anococcygeus. Br J Pharmacol. 1992 Nov;107(3):715–721. doi: 10.1111/j.1476-5381.1992.tb14512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordge M. P., Meyer D. J., Hothersall J., Neild G. H., Payne N. N., Noronha-Dutra A. Copper chelation-induced reduction of the biological activity of S-nitrosothiols. Br J Pharmacol. 1995 Mar;114(5):1083–1089. doi: 10.1111/j.1476-5381.1995.tb13317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann M. A., Castelli I., Pargger H., Drop L. J. Nitric oxide dose-response study in the isolated perfused rat kidney after inhibition of endothelium-derived relaxing factor synthesis: the role of serum albumin. J Pharmacol Exp Ther. 1995 May;273(2):855–862. [PubMed] [Google Scholar]
- Kelm M., Feelisch M., Spahr R., Piper H. M., Noack E., Schrader J. Quantitative and kinetic characterization of nitric oxide and EDRF released from cultured endothelial cells. Biochem Biophys Res Commun. 1988 Jul 15;154(1):236–244. doi: 10.1016/0006-291x(88)90675-4. [DOI] [PubMed] [Google Scholar]
- Kharitonov V. G., Sundquist A. R., Sharma V. S. Kinetics of nitrosation of thiols by nitric oxide in the presence of oxygen. J Biol Chem. 1995 Nov 24;270(47):28158–28164. doi: 10.1074/jbc.270.47.28158. [DOI] [PubMed] [Google Scholar]
- Kowaluk E. A., Fung H. L. Spontaneous liberation of nitric oxide cannot account for in vitro vascular relaxation by S-nitrosothiols. J Pharmacol Exp Ther. 1990 Dec;255(3):1256–1264. [PubMed] [Google Scholar]
- Langford E. J., Brown A. S., Wainwright R. J., de Belder A. J., Thomas M. R., Smith R. E., Radomski M. W., Martin J. F., Moncada S. Inhibition of platelet activity by S-nitrosoglutathione during coronary angioplasty. Lancet. 1994 Nov 26;344(8935):1458–1460. doi: 10.1016/s0140-6736(94)90287-9. [DOI] [PubMed] [Google Scholar]
- Lieberman E. H., O'Neill S., Mendelsohn M. E. S-nitrosocysteine inhibition of human platelet secretion is correlated with increases in platelet cGMP levels. Circ Res. 1991 Jun;68(6):1722–1728. doi: 10.1161/01.res.68.6.1722. [DOI] [PubMed] [Google Scholar]
- Mathews W. R., Kerr S. W. Biological activity of S-nitrosothiols: the role of nitric oxide. J Pharmacol Exp Ther. 1993 Dec;267(3):1529–1537. [PubMed] [Google Scholar]
- Mellion B. T., Ignarro L. J., Myers C. B., Ohlstein E. H., Ballot B. A., Hyman A. L., Kadowitz P. J. Inhibition of human platelet aggregation by S-nitrosothiols. Heme-dependent activation of soluble guanylate cyclase and stimulation of cyclic GMP accumulation. Mol Pharmacol. 1983 May;23(3):653–664. [PubMed] [Google Scholar]
- Meyer D. J., Kramer H., Ozer N., Coles B., Ketterer B. Kinetics and equilibria of S-nitrosothiol-thiol exchange between glutathione, cysteine, penicillamines and serum albumin. FEBS Lett. 1994 May 30;345(2-3):177–180. doi: 10.1016/0014-5793(94)00429-3. [DOI] [PubMed] [Google Scholar]
- Park J. W. Reaction of S-nitrosoglutathione with sulfhydryl groups in protein. Biochem Biophys Res Commun. 1988 Apr 29;152(2):916–920. doi: 10.1016/s0006-291x(88)80127-x. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Rees D. D., Dutra A., Moncada S. S-nitroso-glutathione inhibits platelet activation in vitro and in vivo. Br J Pharmacol. 1992 Nov;107(3):745–749. doi: 10.1111/j.1476-5381.1992.tb14517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfstein J. S., Keaney J. F., Jr, Slivka A., Welch G. N., Vita J. A., Stamler J. S., Loscalzo J. In vivo transfer of nitric oxide between a plasma protein-bound reservoir and low molecular weight thiols. J Clin Invest. 1994 Oct;94(4):1432–1439. doi: 10.1172/JCI117480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton D. J., Muruganandam A., McKenney D. J., Mutus B. Visible light photochemical release of nitric oxide from S-nitrosoglutathione: potential photochemotherapeutic applications. Photochem Photobiol. 1994 Apr;59(4):463–467. doi: 10.1111/j.1751-1097.1994.tb05065.x. [DOI] [PubMed] [Google Scholar]
- Sexton D. J., Mutus B. Platelet glutathione transport: characteristics and evidence for regulation by intraplatelet thiol status. Biochem Cell Biol. 1995 Mar-Apr;73(3-4):155–162. doi: 10.1139/o95-019. [DOI] [PubMed] [Google Scholar]
- Simon D. I., Stamler J. S., Jaraki O., Keaney J. F., Osborne J. A., Francis S. A., Singel D. J., Loscalzo J. Antiplatelet properties of protein S-nitrosothiols derived from nitric oxide and endothelium-derived relaxing factor. Arterioscler Thromb. 1993 Jun;13(6):791–799. doi: 10.1161/01.atv.13.6.791. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Jaraki O., Osborne J., Simon D. I., Keaney J., Vita J., Singel D., Valeri C. R., Loscalzo J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J. S., Simon D. I., Osborne J. A., Mullins M. E., Jaraki O., Michel T., Singel D. J., Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink D. A., Nims R. W., Darbyshire J. F., Christodoulou D., Hanbauer I., Cox G. W., Laval F., Laval J., Cook J. A., Krishna M. C. Reaction kinetics for nitrosation of cysteine and glutathione in aerobic nitric oxide solutions at neutral pH. Insights into the fate and physiological effects of intermediates generated in the NO/O2 reaction. Chem Res Toxicol. 1994 Jul-Aug;7(4):519–525. doi: 10.1021/tx00040a007. [DOI] [PubMed] [Google Scholar]
- de Belder A. J., MacAllister R., Radomski M. W., Moncada S., Vallance P. J. Effects of S-nitroso-glutathione in the human forearm circulation: evidence for selective inhibition of platelet activation. Cardiovasc Res. 1994 May;28(5):691–694. doi: 10.1093/cvr/28.5.691. [DOI] [PubMed] [Google Scholar]
- de Belder A., Lees C., Martin J., Moncada S., Campbell S. Treatment of HELLP syndrome with nitric oxide donor. Lancet. 1995 Jan 14;345(8942):124–125. doi: 10.1016/s0140-6736(95)90088-8. [DOI] [PubMed] [Google Scholar]


