Abstract
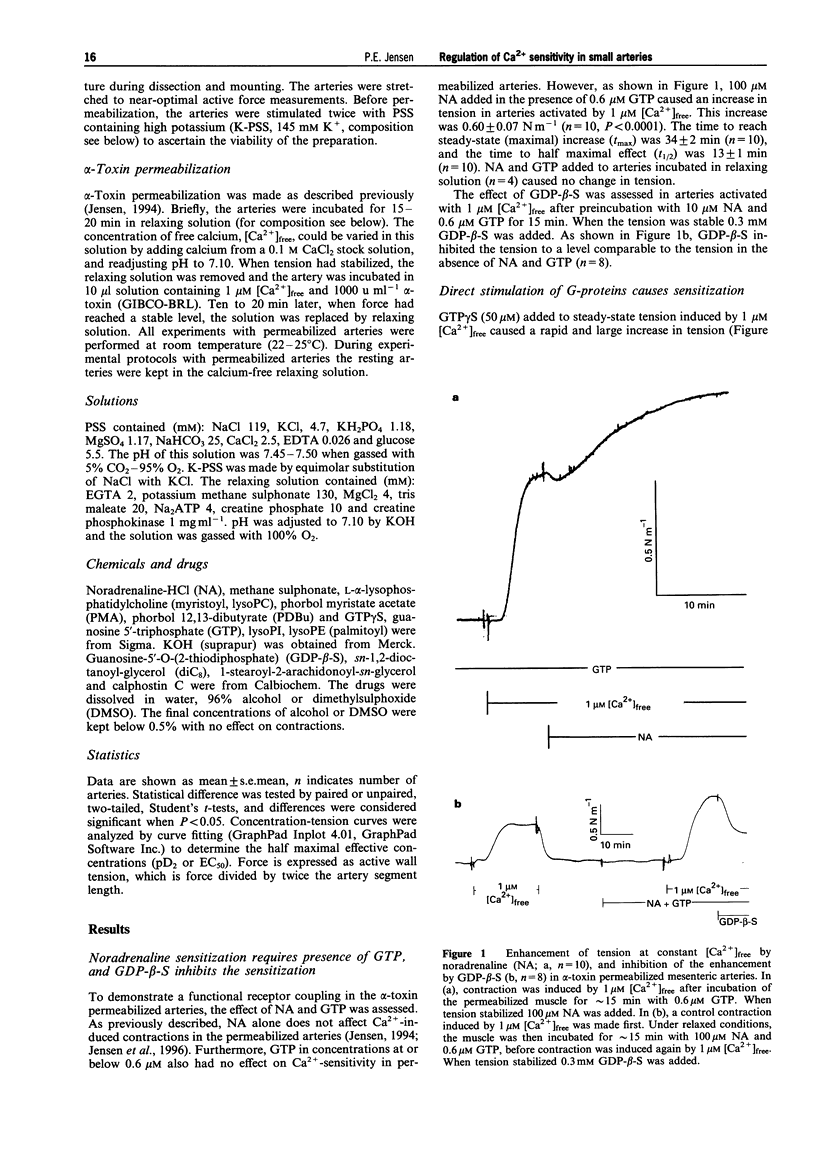
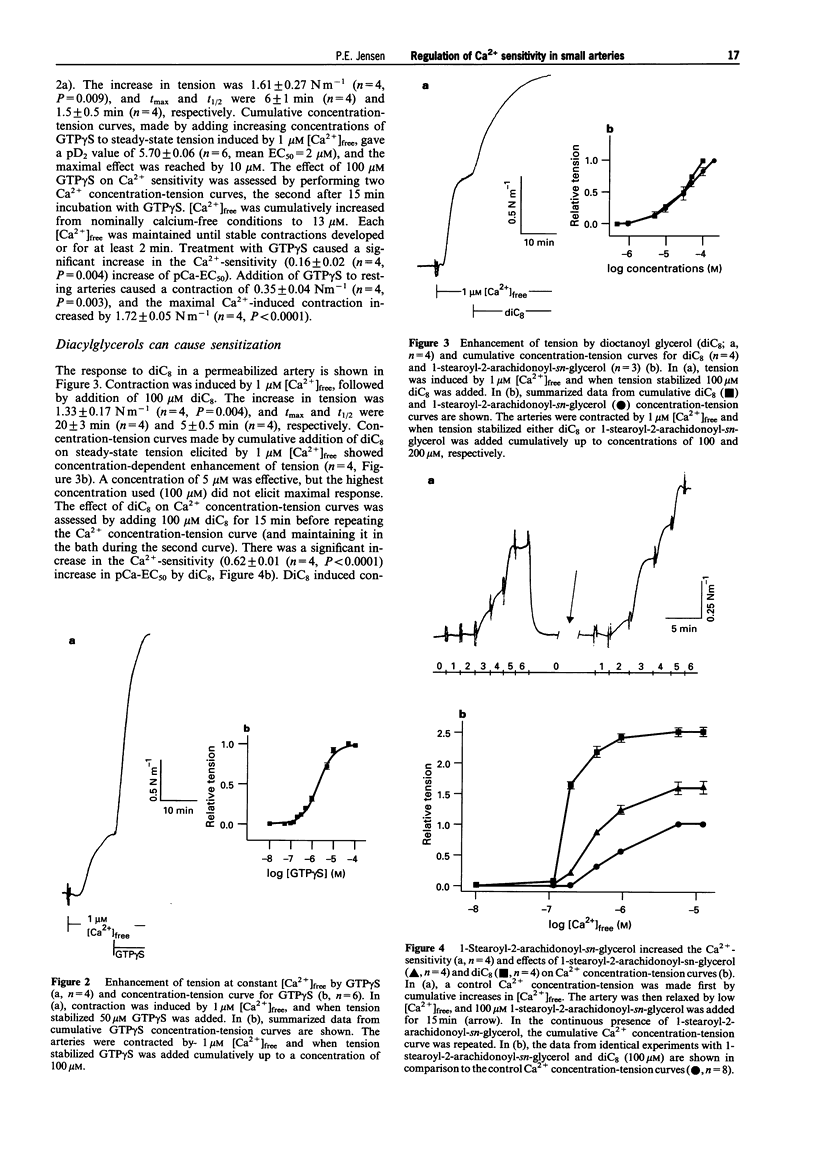
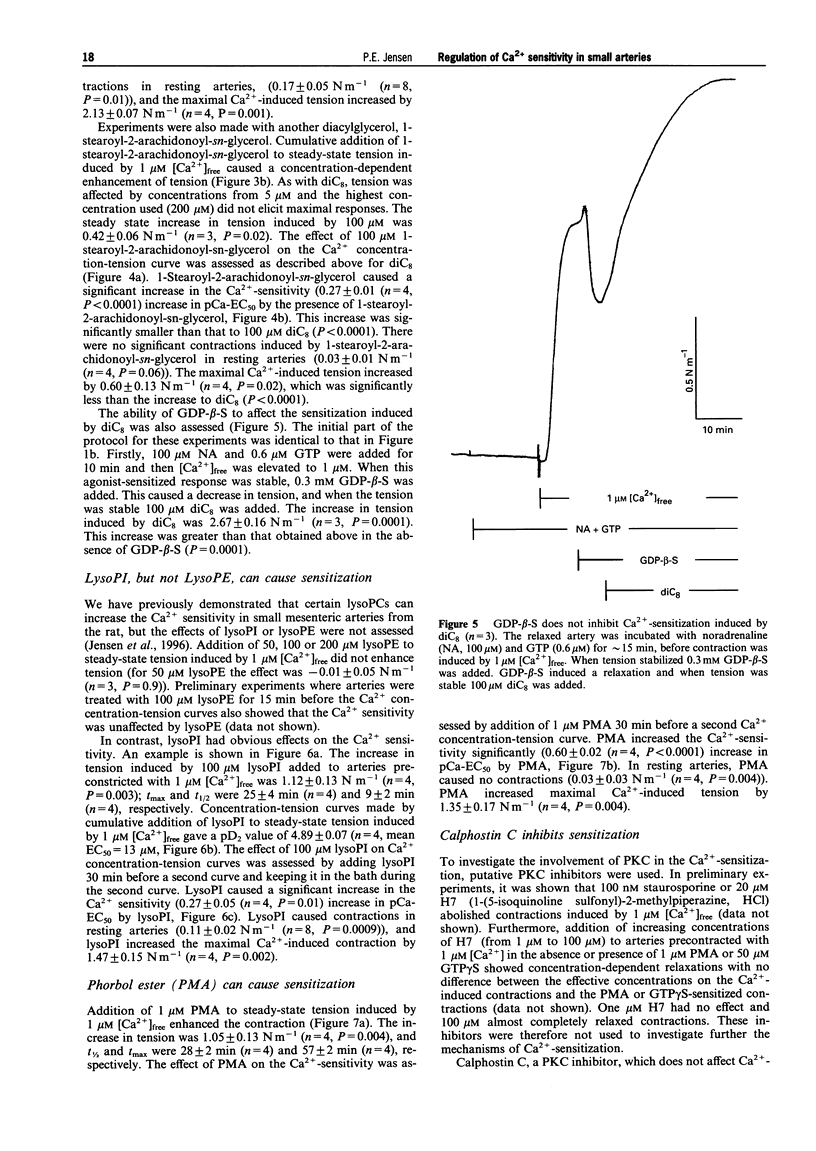
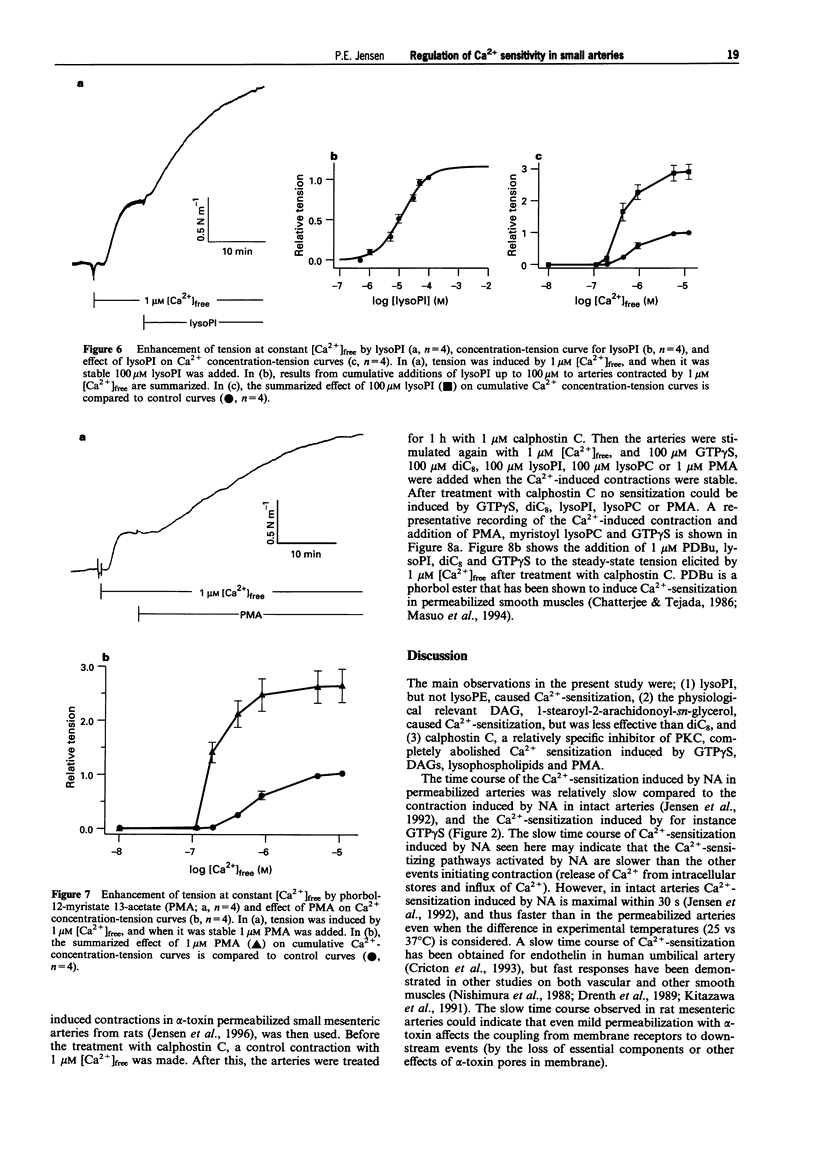
1. A pharmacological characterization was made of the effects of lysophosphatidyl-inositol (lysoPI) and -ethanolamine (lysoPE) on the Ca(2+)-sensitivity of contraction in alpha-toxin permeabilized rat mesenteric arteries. The effect of GTP gamma S (G-protein activator), diacylglycerols (DAGs, dioctanoyl glycerol (diC8) and 1-stearoyl-2-arachidonoyl-sn-glycerol) and phorbol myristate acetate (PMA, protein kinase C (PKC) activator) on Ca(2+)-sensitivity was also assessed. 2. LysoPI increased the Ca(2+)-sensitivity, demonstrated by both an increase in tension induced by 1 microM [Ca2+]free and an increase in the Ca(2+)-sensitivity of Ca2+ concentration-tension curves. LysoPE did not enhance force or Ca(2+)-sensitivity. 3. GTP gamma S enhanced force at constant Ca2+, increased the Ca(2+)-sensitivity, and increased force under Ca(2+)-free conditions. PMA also increased force at constant Ca2+ and increased Ca(2+)-sensitivity, but caused no force development under Ca(2+)-free conditions. 4. DAGs, both diC8 and the more physiological relevant DAG, 1-stearoyl-2-arachidonoyl-sn-glycerol, enhanced force at constant Ca2+ and increased the Ca(2+)-sensitivity. DiC8, in contrast to 1-stearoyl-2-arachidonoyl-sn-glycerol, caused force development under Ca(2+)-free conditions and substantially enhanced force at maximal Ca(2+)-induced contraction. GDP-beta-S abolished the increased Ca(2+)-sensitization induced by noradrenaline, but not that by DAGs. 5. The PKC inhibitor calphostin C completely abolished Ca(2+)-sensitization induced by all of the Ca(2+)-sensitizing agents. 6. These results show that lysoPI can increase the Ca(2+)-sensitivity of smooth muscle contraction, and the Ca(2+)-sensitization induced by DAGs was not completely G-protein mediated, because it was not inhibited by GDP-beta-S. A central role for PKC in regulation of Ca(2+)-sensitization in rat mesenteric small arteries was indicated by the abolishment of Ca(2+)-sensitization by calphostin C.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boonen H. C., De Mey J. G. G-proteins are involved in contractile responses of isolated mesenteric resistance arteries to agonists. Naunyn Schmiedebergs Arch Pharmacol. 1990 Oct;342(4):462–468. doi: 10.1007/BF00169465. [DOI] [PubMed] [Google Scholar]
- Chatterjee M., Tejada M. Phorbol ester-induced contraction in chemically skinned vascular smooth muscle. Am J Physiol. 1986 Sep;251(3 Pt 1):C356–C361. doi: 10.1152/ajpcell.1986.251.3.C356. [DOI] [PubMed] [Google Scholar]
- Crichton C. A., Templeton A. G., McGrath J. C., Smith G. L. Thromboxane A2 analogue, U-46619, potentiates calcium-activated force in human umbilical artery. Am J Physiol. 1993 Jun;264(6 Pt 2):H1878–H1883. doi: 10.1152/ajpheart.1993.264.6.H1878. [DOI] [PubMed] [Google Scholar]
- Fujita A., Takeuchi T., Nakajima H., Nishio H., Hata F. Involvement of heterotrimeric GTP-binding protein and rho protein, but not protein kinase C, in agonist-induced Ca2+ sensitization of skinned muscle of guinea pig vas deferens. J Pharmacol Exp Ther. 1995 Jul;274(1):555–561. [PubMed] [Google Scholar]
- Gong M. C., Fuglsang A., Alessi D., Kobayashi S., Cohen P., Somlyo A. V., Somlyo A. P. Arachidonic acid inhibits myosin light chain phosphatase and sensitizes smooth muscle to calcium. J Biol Chem. 1992 Oct 25;267(30):21492–21498. [PubMed] [Google Scholar]
- Hirata K., Kikuchi A., Sasaki T., Kuroda S., Kaibuchi K., Matsuura Y., Seki H., Saida K., Takai Y. Involvement of rho p21 in the GTP-enhanced calcium ion sensitivity of smooth muscle contraction. J Biol Chem. 1992 May 5;267(13):8719–8722. [PubMed] [Google Scholar]
- Hori M., Sato K., Miyamoto S., Ozaki H., Karaki H. Different pathways of calcium sensitization activated by receptor agonists and phorbol esters in vascular smooth muscle. Br J Pharmacol. 1993 Dec;110(4):1527–1531. doi: 10.1111/j.1476-5381.1993.tb13996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itagaki M., Komori S., Unno T., Syuto B., Ohashi H. Possible involvement of a small G-protein sensitive to exoenzyme C3 of Clostridium botulinum in the regulation of myofilament Ca2+ sensitivity in beta-escin skinned smooth muscle of guinea pig ileum. Jpn J Pharmacol. 1995 Jan;67(1):1–7. doi: 10.1254/jjp.67.1. [DOI] [PubMed] [Google Scholar]
- Itoh T., Suzuki A., Watanabe Y. Effect of a peptide inhibitor of protein kinase C on G-protein-mediated increase in myofilament Ca(2+)-sensitivity in rabbit arterial skinned muscle. Br J Pharmacol. 1994 Jan;111(1):311–317. doi: 10.1111/j.1476-5381.1994.tb14061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. E., Hughes A., Boonen H. C., Aalkjaer C. Force, membrane potential, and [Ca2+]i during activation of rat mesenteric small arteries with norepinephrine, potassium, aluminum fluoride, and phorbol ester. Effects of changes in pHi. Circ Res. 1993 Aug;73(2):314–324. doi: 10.1161/01.res.73.2.314. [DOI] [PubMed] [Google Scholar]
- Jensen P. E., Mulvany M. J., Aalkjaer C. Endogenous and exogenous agonist-induced changes in the coupling between [Ca2+]i and force in rat resistance arteries. Pflugers Arch. 1992 Apr;420(5-6):536–543. doi: 10.1007/BF00374630. [DOI] [PubMed] [Google Scholar]
- Jensen P. E., Ohanian J., Stausbøl-Grøn B., Buus N. H., Aalkjaer C. Increase by lysophosphatidylcholines of smooth muscle Ca2+ sensitivity in alpha-toxin-permeabilized small mesenteric artery from the rat. Br J Pharmacol. 1996 Mar;117(6):1238–1244. doi: 10.1111/j.1476-5381.1996.tb16721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn R. A. Fluoride is not an activator of the smaller (20-25 kDa) GTP-binding proteins. J Biol Chem. 1991 Aug 25;266(24):15595–15597. [PubMed] [Google Scholar]
- Kawase T., Van Breemen C. Aluminum fluoride induces a reversible Ca2+ sensitization in alpha-toxin-permeabilized vascular smooth muscle. Eur J Pharmacol. 1992 Apr 7;214(1):39–44. doi: 10.1016/0014-2999(92)90093-j. [DOI] [PubMed] [Google Scholar]
- Kitazawa T., Gaylinn B. D., Denney G. H., Somlyo A. P. G-protein-mediated Ca2+ sensitization of smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1991 Jan 25;266(3):1708–1715. [PubMed] [Google Scholar]
- Masuo M., Reardon S., Ikebe M., Kitazawa T. A novel mechanism for the Ca(2+)-sensitizing effect of protein kinase C on vascular smooth muscle: inhibition of myosin light chain phosphatase. J Gen Physiol. 1994 Aug;104(2):265–286. doi: 10.1085/jgp.104.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura J., Khalil R. A., Drenth J. P., van Breemen C. Evidence for increased myofilament Ca2+ sensitivity in norepinephrine-activated vascular smooth muscle. Am J Physiol. 1990 Jul;259(1 Pt 2):H2–H8. doi: 10.1152/ajpheart.1990.259.1.H2. [DOI] [PubMed] [Google Scholar]
- Nishimura J., Kolber M., van Breemen C. Norepinephrine and GTP-gamma-S increase myofilament Ca2+ sensitivity in alpha-toxin permeabilized arterial smooth muscle. Biochem Biophys Res Commun. 1988 Dec 15;157(2):677–683. doi: 10.1016/s0006-291x(88)80303-6. [DOI] [PubMed] [Google Scholar]
- Nishimura J., Moreland S., Ahn H. Y., Kawase T., Moreland R. S., van Breemen C. Endothelin increases myofilament Ca2+ sensitivity in alpha-toxin-permeabilized rabbit mesenteric artery. Circ Res. 1992 Oct;71(4):951–959. doi: 10.1161/01.res.71.4.951. [DOI] [PubMed] [Google Scholar]
- Ohanian J., Ollerenshaw J., Collins P., Heagerty A. Agonist-induced production of 1,2-diacylglycerol and phosphatidic acid in intact resistance arteries. Evidence that accumulation of diacylglycerol is not a prerequisite for contraction. J Biol Chem. 1990 May 25;265(15):8921–8928. [PubMed] [Google Scholar]
- Oishi K., Raynor R. L., Charp P. A., Kuo J. F. Regulation of protein kinase C by lysophospholipids. Potential role in signal transduction. J Biol Chem. 1988 May 15;263(14):6865–6871. [PubMed] [Google Scholar]
- Rapoport R. M., Campbell A. K., Bazan E. Effects of PKC downregulation on norepinephrine- and prostaglandin F2 alpha-induced contraction in rat aorta. Am J Physiol. 1995 Aug;269(2 Pt 2):H590–H598. doi: 10.1152/ajpheart.1995.269.2.H590. [DOI] [PubMed] [Google Scholar]
- Ruzycky A. L., Morgan K. G. Involvement of the protein kinase C system in calcium-force relationships in ferret aorta. Br J Pharmacol. 1989 Jun;97(2):391–400. doi: 10.1111/j.1476-5381.1989.tb11966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh S., Rensland H., Pfitzer G. Ras proteins increase Ca(2+)-responsiveness of smooth muscle contraction. FEBS Lett. 1993 Jun 14;324(2):211–215. doi: 10.1016/0014-5793(93)81395-g. [DOI] [PubMed] [Google Scholar]
- Sohn U. D., Chiu T. T., Bitar K. N., Hillemeier C., Behar J., Biancani P. Calcium requirements for acetylcholine-induced contraction of cat esophageal circular muscle cells. Am J Physiol. 1994 Feb;266(2 Pt 1):G330–G338. doi: 10.1152/ajpgi.1994.266.2.G330. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V. Signal transduction and regulation in smooth muscle. Nature. 1994 Nov 17;372(6503):231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- Stabel S., Parker P. J. Protein kinase C. Pharmacol Ther. 1991;51(1):71–95. doi: 10.1016/0163-7258(91)90042-k. [DOI] [PubMed] [Google Scholar]
- Ward D. T., Ohanian J., Heagerty A. M., Ohanian V. Phospholipase D-induced phosphatidate production in intact small arteries during noradrenaline stimulation: involvement of both G-protein and tyrosine-phosphorylation-linked pathways. Biochem J. 1995 Apr 15;307(Pt 2):451–456. doi: 10.1042/bj3070451. [DOI] [PMC free article] [PubMed] [Google Scholar]


