Abstract
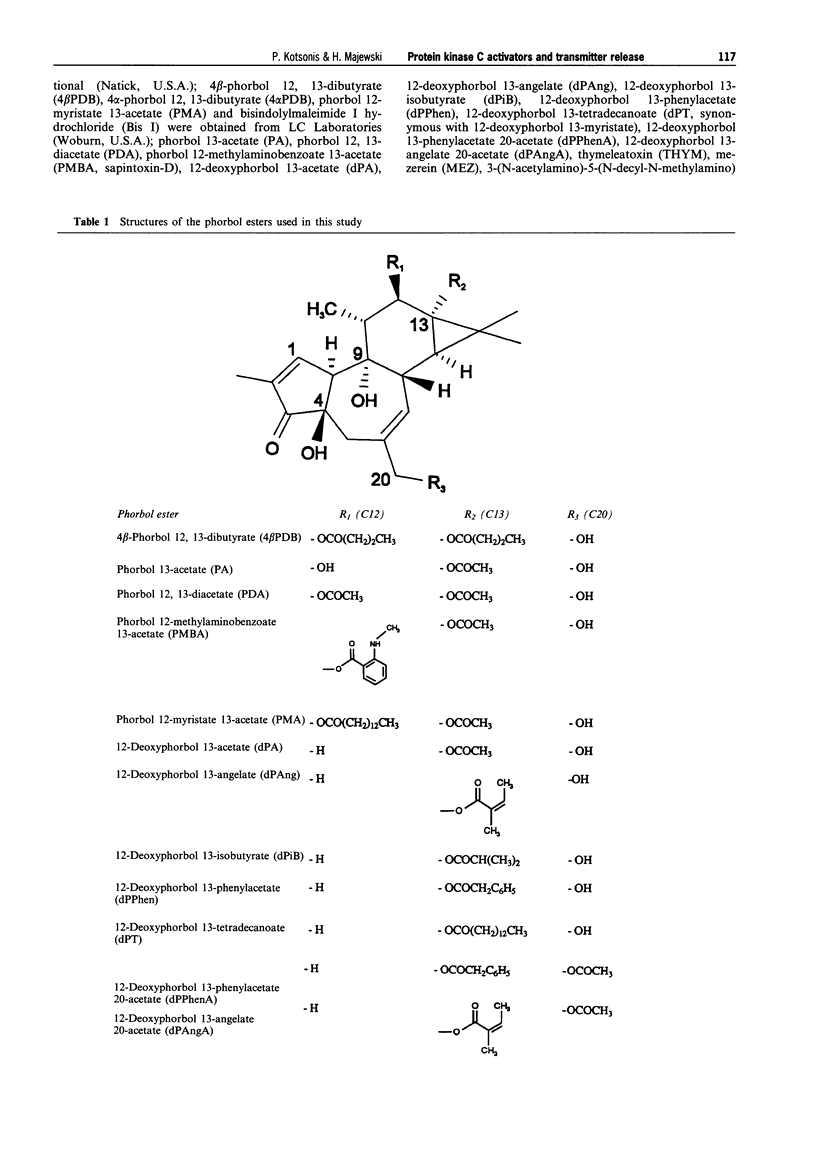
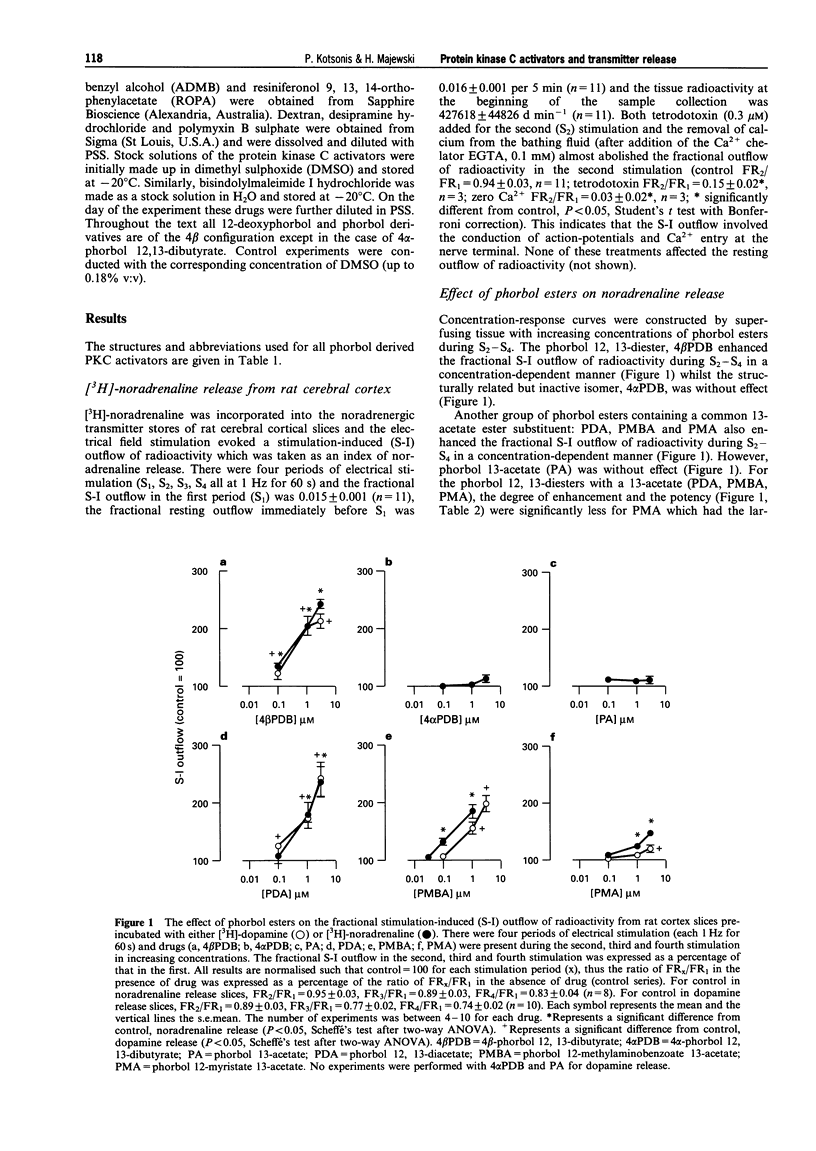
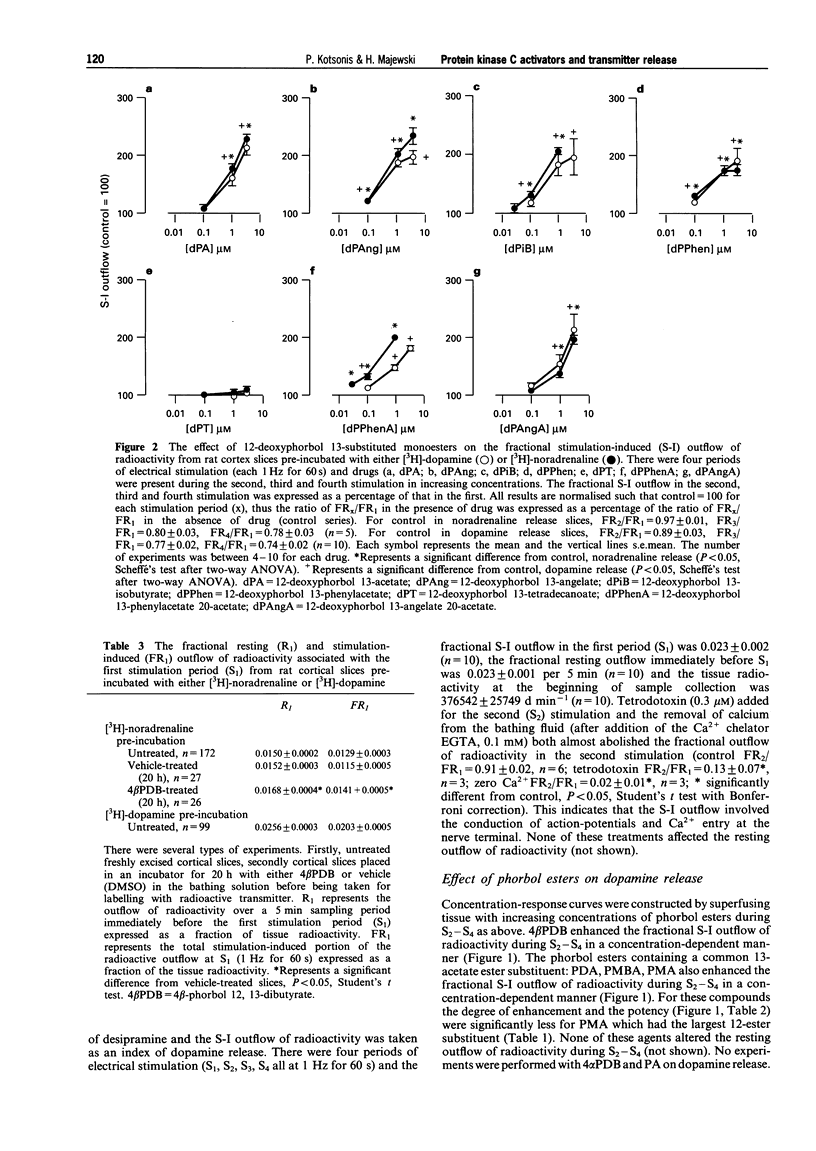
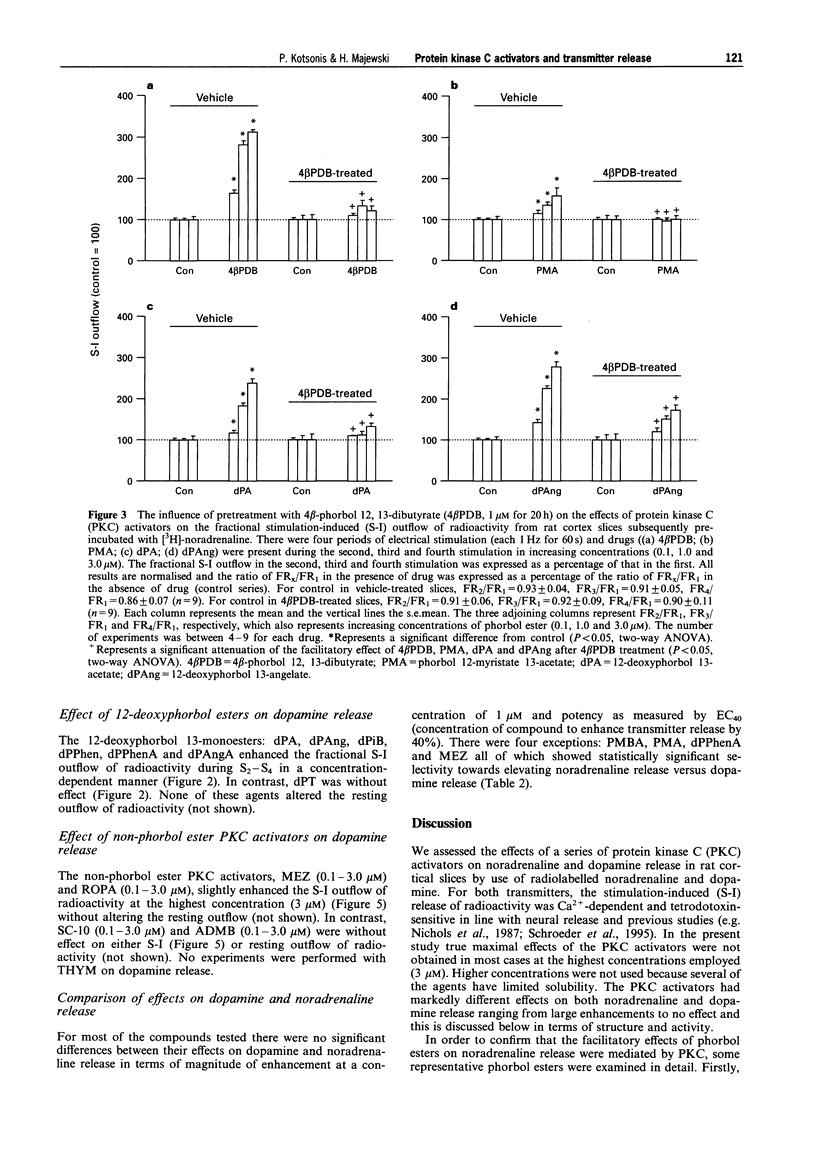
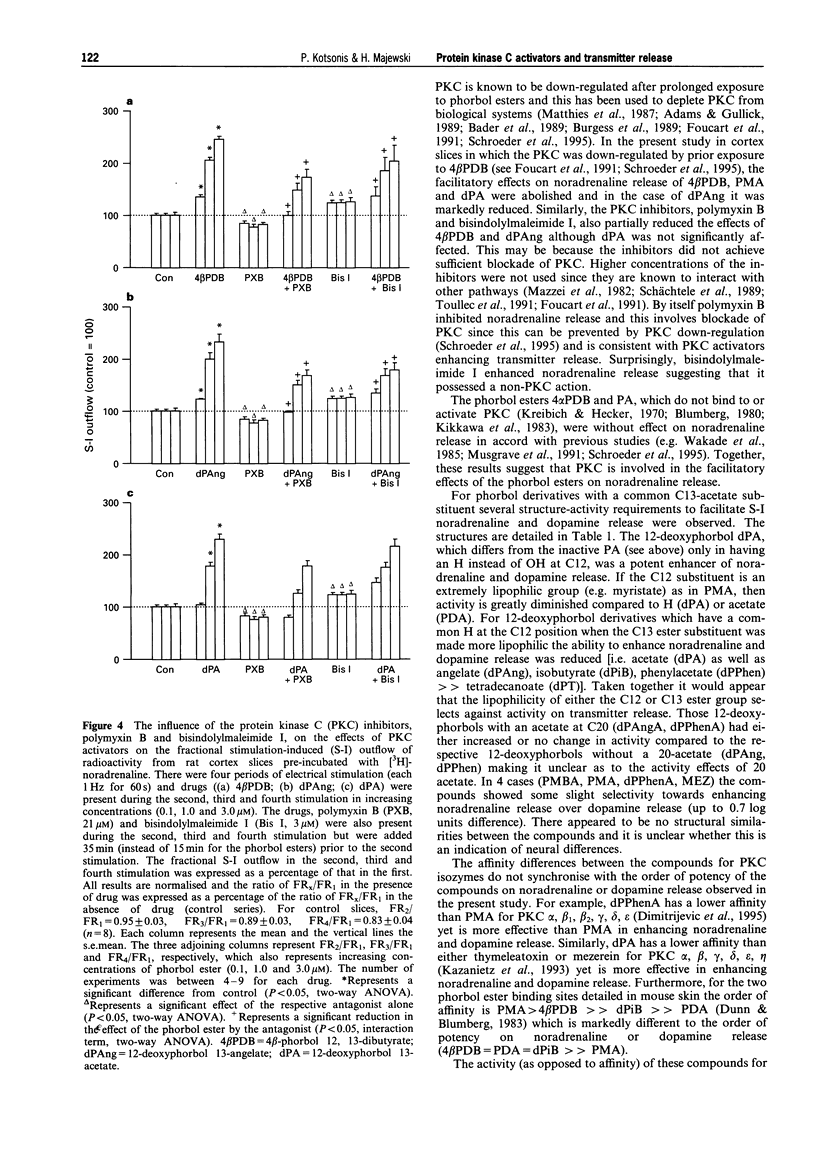
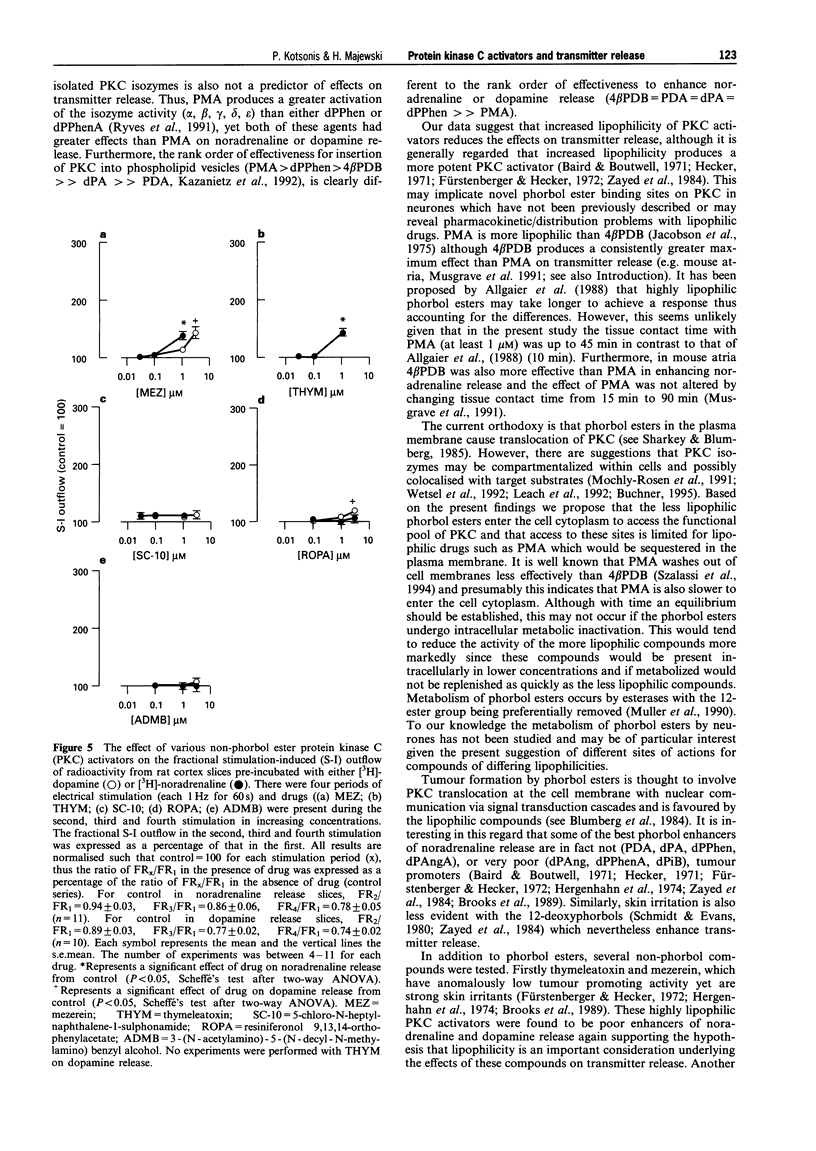
1. The effects of various protein kinase C (PKC) activators on the stimulation-induced (S-I) release of noradrenaline and dopamine was studied in rat cortical slices pre-incubated with [3H]-noradrenaline or [3H]-dopamine. The aim was to investigate a possible structure-activity relationship for these agents on transmitter release. 2. 4 beta-Phorbol 12,13-dibutyrate (4 beta PDB, 0.1-3.0 microM), enhanced S-I noradrenaline and dopamine release in a concentration-dependent manner whereas the structurally related inactive isomer 4 alpha-phorbol 12, 13-dibutyrate (4 alpha PDB, 0.1-3.0 microM) and phorbol 13-acetate (PA, 0.1-3.0microM) were without effect on noradrednaline release. Another group of phorbol 12, 13-diesters containing a common 13-ester substituent (phorbol 12, 13-diacetate, PDA, 0.1-3.0 microM; phorbol 12-myristate 13-acetate, PMA, 0.1-3.0 microM; phorbol 12-methylaminobenzoate 13-acetate, PMBA, 0.03-3.0 microM) also enhanced S-I noradrenaline and dopamine release in a concentration-dependent manner with PMA being the least potent. 3. The 12-deoxyphorbol 13-substituted monoesters, 12-deoxyphorbol 13-acetate (dPA, 0.1-3.0 microM), 12-deoxyphorbol 13-angelate (dPAng, 0.1-3.0 microM), 12-deoxyphorbol 13-isobutyrate (dPiB, 0.03-3.0 microM) and 12-deoxyphorbol 13-phenylacetate (dPPhen, 0.1-3.0 microM) enhanced S-I noradrenaline and dopamine release in a concentration-dependent manner. In contrast, 12-deoxyphorbol 13-tetradecanoate (dPT, 0.1-3.0 microM) was without effect. 4. The involvement of PKC in mediating the effects of the various phorbol esters was further investigated. PKC was down-regulated by 20 h exposure of the cortical slices to 4 beta-phorbol 12,13-dibutyrate (1 microM). In this case the facilitatory effect of 4 beta PDB and dPA was abolished whilst that of dPAng was significantly attenuated. This indicates that these agents were acting selectively at PKC. In support of this the PKC inhibitors, polymyxin B (21 microM) and bisindolylmaleimide I (3 microM), attenuated the facilitatory effect of 4 beta PDB and dPAng although that of dPA was not significantly altered. 5. The effects of these agents on transmitter release were not correlated with their in vitro affinity and isozyme selectivity for PKC. Short chain substituted mono- and diesters of phorbol were more potent enhancers of action-potential evoked noradrenaline and dopamine release than the long chain esters. Interestingly, these former agents are the least potent or non effective (e.g. dPA, PDA) tumour promoters. We suggest that the reason for the poor effects of lipophilic long chain phorbol esters (PMA, dPT) on transmitter release is that they are sequestered in the plasmalemma and do not access the cell cytoplasm where the PKC may be located.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C., Gullick W. J. Differences in phorbol-ester-induced down-regulation of protein kinase C between cell lines. Biochem J. 1989 Feb 1;257(3):905–911. doi: 10.1042/bj2570905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allgaier C., Daschmann B., Huang H. Y., Hertting G. Protein kinase C and presynaptic modulation of acetylcholine release in rabbit hippocampus. Br J Pharmacol. 1988 Mar;93(3):525–534. doi: 10.1111/j.1476-5381.1988.tb10307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allgaier C., Hertting G., Huang H. Y., Jackisch R. Protein kinase C activation and alpha 2-autoreceptor-modulated release of noradrenaline. Br J Pharmacol. 1987 Sep;92(1):161–172. doi: 10.1111/j.1476-5381.1987.tb11308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader M. F., Sontag J. M., Thiersé D., Aunis D. A reassessment of guanine nucleotide effects on catecholamine secretion from permeabilized adrenal chromaffin cells. J Biol Chem. 1989 Oct 5;264(28):16426–16434. [PubMed] [Google Scholar]
- Baird W. M., Boutwell R. K. Tumor-promoting activity of phorbol and four diesters of phorbol in mouse skin. Cancer Res. 1971 Aug;31(8):1074–1079. [PubMed] [Google Scholar]
- Bell R. M., Burns D. J. Lipid activation of protein kinase C. J Biol Chem. 1991 Mar 15;266(8):4661–4664. [PubMed] [Google Scholar]
- Blumberg P. M. In vitro studies on the mode of action of the phorbol esters, potent tumor promoters: part 1. Crit Rev Toxicol. 1980 Dec;8(2):153–197. doi: 10.3109/10408448009037493. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M., Jaken S., König B., Sharkey N. A., Leach K. L., Jeng A. Y., Yeh E. Mechanism of action of the phorbol ester tumor promoters: specific receptors for lipophilic ligands. Biochem Pharmacol. 1984 Mar 15;33(6):933–940. doi: 10.1016/0006-2952(84)90448-9. [DOI] [PubMed] [Google Scholar]
- Brooks G., Evans A. T., Aitken A., Evans F. J. Tumour-promoting and hyperplastic effects of phorbol and daphnane esters in CD-1 mouse skin and a synergistic effect of calcium ionophore with the non-promoting activator of protein kinase C, sapintoxin A. Carcinogenesis. 1989 Feb;10(2):283–288. doi: 10.1093/carcin/10.2.283. [DOI] [PubMed] [Google Scholar]
- Buchner K. Protein kinase C in the transduction of signals toward and within the cell nucleus. Eur J Biochem. 1995 Mar 1;228(2):211–221. [PubMed] [Google Scholar]
- Burgess G. M., Mullaney I., McNeill M., Dunn P. M., Rang H. P. Second messengers involved in the mechanism of action of bradykinin in sensory neurons in culture. J Neurosci. 1989 Sep;9(9):3314–3325. doi: 10.1523/JNEUROSCI.09-09-03314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daschmann B., Allgaier C., Nakov R., Hertting G. Staurosporine counteracts the phorbol ester-induced enhancement of neurotransmitter release in hippocampus. Arch Int Pharmacodyn Ther. 1988 Nov-Dec;296:232–245. [PubMed] [Google Scholar]
- Dekker L. V., Parker P. J. Protein kinase C--a question of specificity. Trends Biochem Sci. 1994 Feb;19(2):73–77. doi: 10.1016/0968-0004(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Dimitrijević S. M., Ryves W. J., Parker P. J., Evans F. J. Characterization of phorbol ester binding to protein kinase C isotypes. Mol Pharmacol. 1995 Aug;48(2):259–267. [PubMed] [Google Scholar]
- Dunn J. A., Blumberg P. M. Specific binding of [20-3H]12-deoxyphorbol 13-isobutyrate to phorbol ester receptor subclasses in mouse skin particulate preparations. Cancer Res. 1983 Oct;43(10):4632–4637. [PubMed] [Google Scholar]
- Evans F. J., Parker P. J., Olivier A. R., Thomas S., Ryves W. J., Evans A. T., Gordge P., Sharma P. Phorbol ester activation of the isotypes of protein kinase C from bovine and rat brain. Biochem Soc Trans. 1991 Apr;19(2):397–402. doi: 10.1042/bst0190397. [DOI] [PubMed] [Google Scholar]
- Fürstenberger G., Hecker E. Zum Wirkungsmechanismus cocarcinogener Pflanzeninhaltsstoffe. Planta Med. 1972 Nov;22(3):241–266. doi: 10.1055/s-0028-1099610. [DOI] [PubMed] [Google Scholar]
- Hergenhahn M., Kusumoto S., Hecker E. Diterpene esters from 'Euphorbium' and their irritant and cocarcinogenic activity. Experientia. 1974 Dec 15;30(12):1438–1440. doi: 10.1007/BF01919683. [DOI] [PubMed] [Google Scholar]
- Hoffmann I. S., Talmaciu R. K., Ferro C. P., Cubeddu L. X. Sustained high release at rapid stimulation rates and reduced functional autoreceptors characterize prefrontal cortex dopamine terminals. J Pharmacol Exp Ther. 1988 Jun;245(3):761–772. [PubMed] [Google Scholar]
- Ito M., Tanaka T., Inagaki M., Nakanishi K., Hidaka H. N-(6-phenylhexyl)-5-chloro-1-naphthalenesulfonamide, a novel activator of protein kinase C. Biochemistry. 1986 Jul 29;25(15):4179–4184. doi: 10.1021/bi00363a002. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Wenner C. E., Kemp G., Papahadjopoulos D. Surface properties of phorbol esters and their interaction with lipid monolayers and bilayers. Cancer Res. 1975 Nov;35(11 Pt 1):2991–2995. [PubMed] [Google Scholar]
- Kazanietz M. G., Areces L. B., Bahador A., Mischak H., Goodnight J., Mushinski J. F., Blumberg P. M. Characterization of ligand and substrate specificity for the calcium-dependent and calcium-independent protein kinase C isozymes. Mol Pharmacol. 1993 Aug;44(2):298–307. [PubMed] [Google Scholar]
- Kazanietz M. G., Krausz K. W., Blumberg P. M. Differential irreversible insertion of protein kinase C into phospholipid vesicles by phorbol esters and related activators. J Biol Chem. 1992 Oct 15;267(29):20878–20886. [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Tanaka Y., Miyake R., Nishizuka Y. Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. J Biol Chem. 1983 Oct 10;258(19):11442–11445. [PubMed] [Google Scholar]
- Kreibich G., Hecker E. Active principles of croton oil. X. Preparation of tritium labeled croton oil factor A1 and other tritium labeled phorbol derivatives. Z Krebsforsch. 1970;74(4):448–456. [PubMed] [Google Scholar]
- Leach K. L., Ruff V. A., Jarpe M. B., Adams L. D., Fabbro D., Raben D. M. Alpha-thrombin stimulates nuclear diglyceride levels and differential nuclear localization of protein kinase C isozymes in IIC9 cells. J Biol Chem. 1992 Oct 25;267(30):21816–21822. [PubMed] [Google Scholar]
- Matthies H. J., Palfrey H. C., Hirning L. D., Miller R. J. Down regulation of protein kinase C in neuronal cells: effects on neurotransmitter release. J Neurosci. 1987 Apr;7(4):1198–1206. doi: 10.1523/JNEUROSCI.07-04-01198.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzei G. J., Katoh N., Kuo J. F. Polymyxin B is a more selective inhibitor for phospholipid-sensitive Ca2+-dependent protein kinase than for calmodulin-sensitive Ca2+-dependent protein kinase. Biochem Biophys Res Commun. 1982 Dec 31;109(4):1129–1133. doi: 10.1016/0006-291x(82)91894-0. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D., Khaner H., Lopez J., Smith B. L. Intracellular receptors for activated protein kinase C. Identification of a binding site for the enzyme. J Biol Chem. 1991 Aug 15;266(23):14866–14868. [PubMed] [Google Scholar]
- Musgrave I. F., Foucart S., Majewski H. Evidence that angiotensin II enhances noradrenaline release from sympathetic nerves in mouse atria by activating protein kinase C. J Auton Pharmacol. 1991 Aug;11(4):211–220. doi: 10.1111/j.1474-8673.1991.tb00319.x. [DOI] [PubMed] [Google Scholar]
- Musgrave I. F., Majewski H. Effect of phorbol esters and polymyxin B on modulation of noradrenaline release in mouse atria. Naunyn Schmiedebergs Arch Pharmacol. 1989 Jan-Feb;339(1-2):48–53. doi: 10.1007/BF00165125. [DOI] [PubMed] [Google Scholar]
- Müller G., Hergenhahn M., Roeser H., Tremp G. L., Schmidt R., Hecker E. Toxicokinetics of tumor promoters of mouse skin. I. Metabolism of phorbol and ingenol esters by mouse liver microsomes. Carcinogenesis. 1990 Jul;11(7):1127–1132. doi: 10.1093/carcin/11.7.1127. [DOI] [PubMed] [Google Scholar]
- Nichols R. A., Haycock J. W., Wang J. K., Greengard P. Phorbol ester enhancement of neurotransmitter release from rat brain synaptosomes. J Neurochem. 1987 Feb;48(2):615–621. doi: 10.1111/j.1471-4159.1987.tb04137.x. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Ryves W. J., Evans A. T., Olivier A. R., Parker P. J., Evans F. J. Activation of the PKC-isotypes alpha, beta 1, gamma, delta and epsilon by phorbol esters of different biological activities. FEBS Lett. 1991 Aug 19;288(1-2):5–9. doi: 10.1016/0014-5793(91)80989-g. [DOI] [PubMed] [Google Scholar]
- Schmidt R. J., Evans F. J. Skin irritant effects of esters of phorbol and related polyols. Arch Toxicol. 1980 Apr;44(4):279–289. doi: 10.1007/BF00278035. [DOI] [PubMed] [Google Scholar]
- Schroeder G. E., Kotsonis P., Musgrave I. F., Majewski H. Protein kinase C involvement in maintenance and modulation of noradrenaline release in the mouse brain cortex. Br J Pharmacol. 1995 Nov;116(6):2757–2763. doi: 10.1111/j.1476-5381.1995.tb17238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szallasi Z., Smith C. B., Blumberg P. M. Dissociation of phorbol esters leads to immediate redistribution to the cytosol of protein kinases C alpha and C delta in mouse keratinocytes. J Biol Chem. 1994 Nov 4;269(44):27159–27162. [PubMed] [Google Scholar]
- Takata Y., Ozawa J., Kato H. Presynaptic sites of isolated canine saphenous veins are more sensitive to protein kinase C than postsynaptic ones. Jpn J Pharmacol. 1991 Jul;56(3):337–348. doi: 10.1254/jjp.56.337. [DOI] [PubMed] [Google Scholar]
- Toullec D., Pianetti P., Coste H., Bellevergue P., Grand-Perret T., Ajakane M., Baudet V., Boissin P., Boursier E., Loriolle F. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991 Aug 25;266(24):15771–15781. [PubMed] [Google Scholar]
- Versteeg D. H., Ulenkate H. J. Basal and electrically stimulated release of [3H]noradrenaline and [3H]dopamine from rat amygdala slices in vitro: effects of 4 beta-phorbol 12,13-dibutyrate, 4 alpha-phorbol 12,13-didecanoate and polymyxin B. Brain Res. 1987 Jul 28;416(2):343–348. doi: 10.1016/0006-8993(87)90916-4. [DOI] [PubMed] [Google Scholar]
- Wakade A. R., Malhotra R. K., Wakade T. D. Phorbol ester, an activator of protein kinase C, enhances calcium-dependent release of sympathetic neurotransmitter. Naunyn Schmiedebergs Arch Pharmacol. 1985 Oct;331(1):122–124. doi: 10.1007/BF00498863. [DOI] [PubMed] [Google Scholar]
- Wender P. A., Koehler K. F., Sharkey N. A., Dell'Aquila M. L., Blumberg P. M. Analysis of the phorbol ester pharmacophore on protein kinase C as a guide to the rational design of new classes of analogs. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4214–4218. doi: 10.1073/pnas.83.12.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetsel W. C., Khan W. A., Merchenthaler I., Rivera H., Halpern A. E., Phung H. M., Negro-Vilar A., Hannun Y. A. Tissue and cellular distribution of the extended family of protein kinase C isoenzymes. J Cell Biol. 1992 Apr;117(1):121–133. doi: 10.1083/jcb.117.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zayed S., Sorg B., Hecker E. Structure activity relations of polyfunctional diterpenes of the tigliane type, VI. Irritant and tumor promoting activities of semisynthetic mono and diesters of 12-deoxyphorbol. Planta Med. 1984 Feb;50(1):65–69. [PubMed] [Google Scholar]


