Abstract
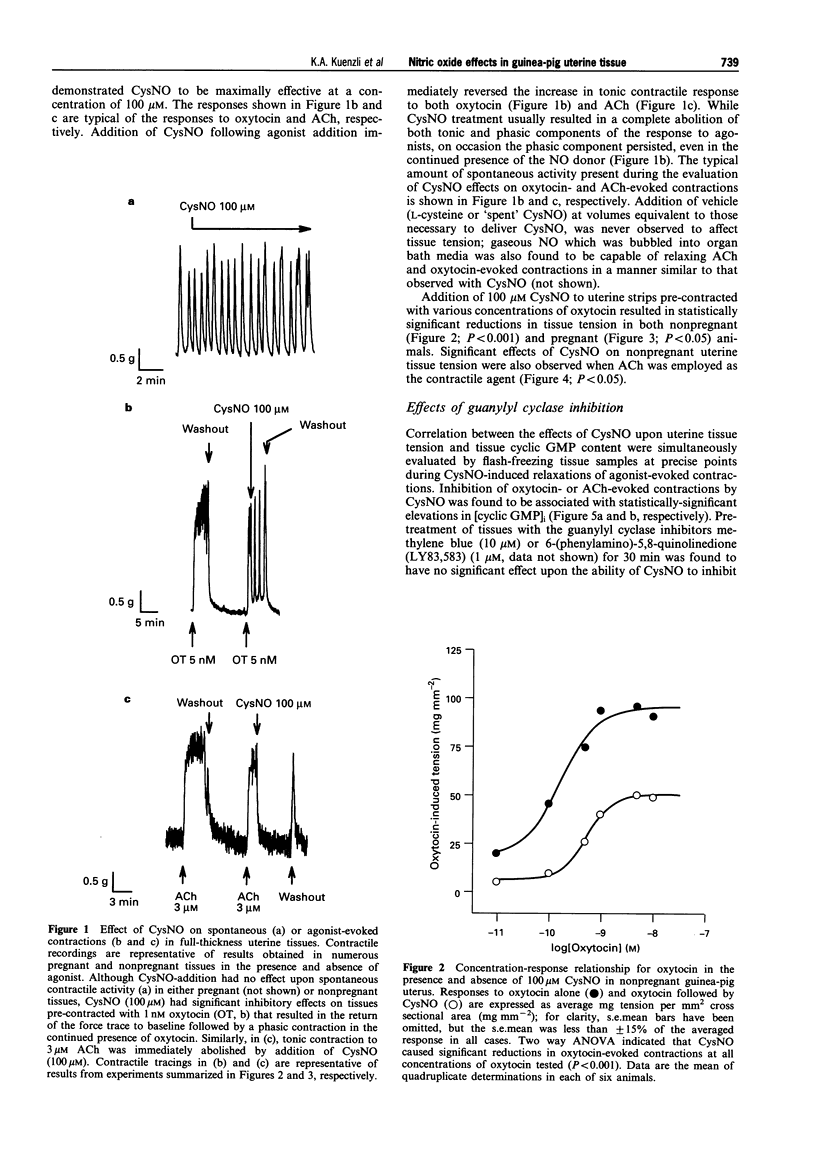
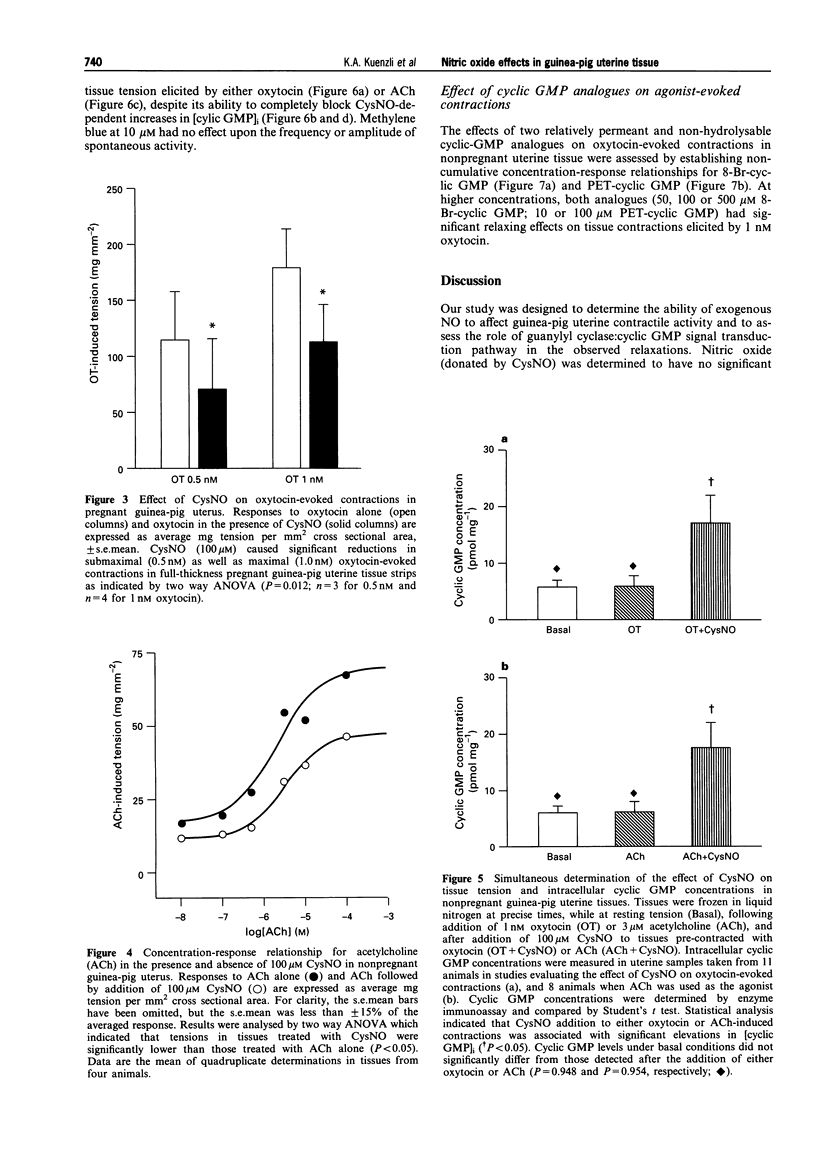
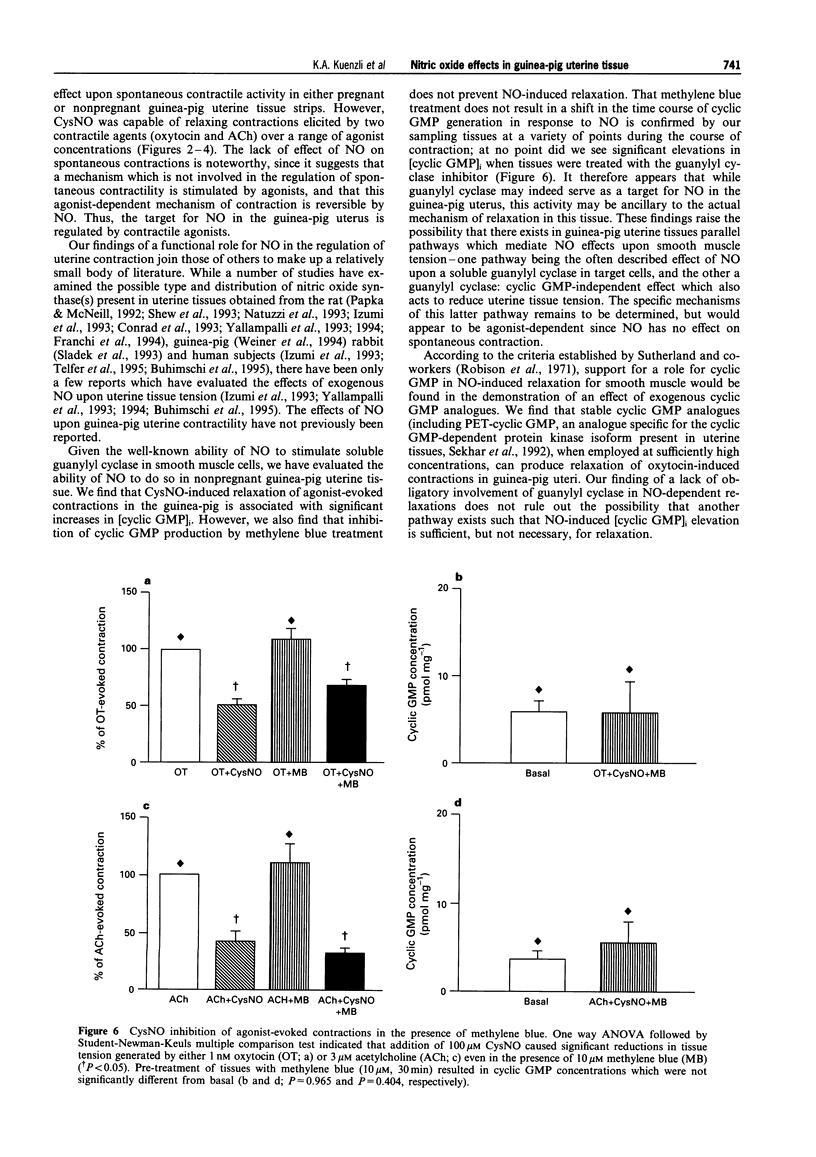
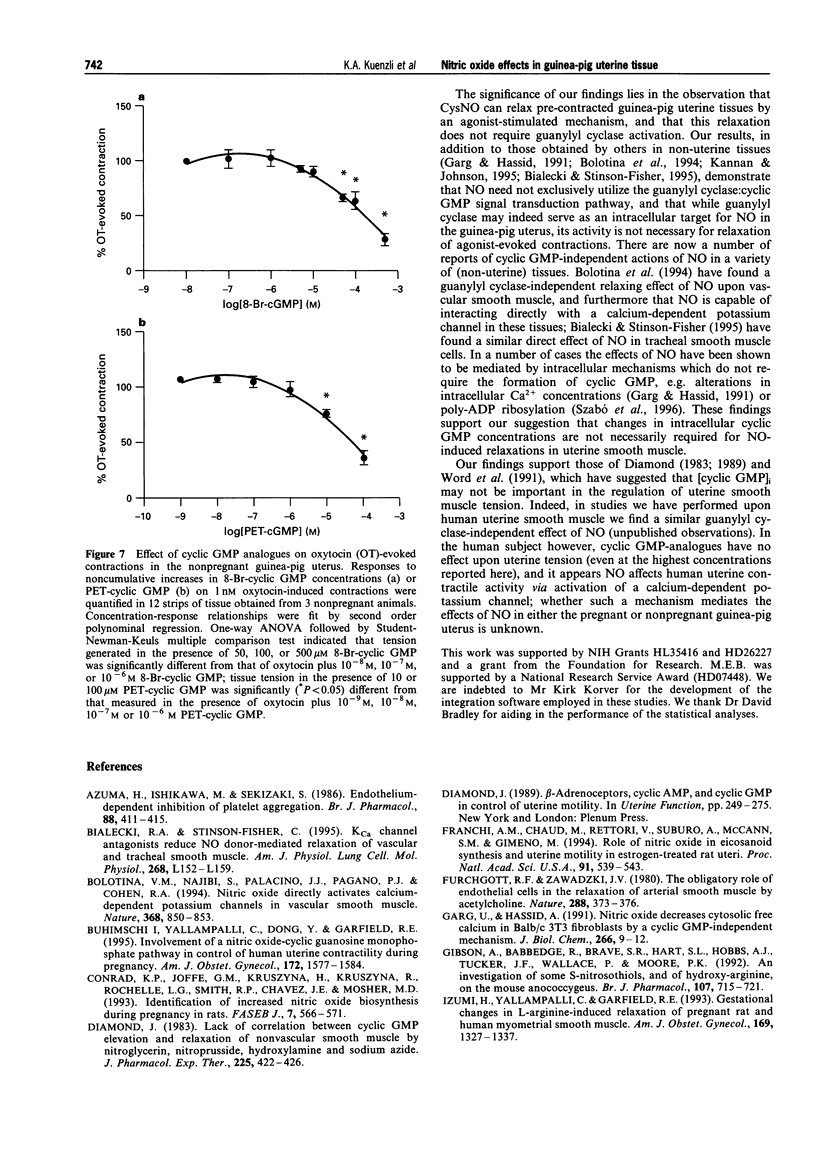
1. The role of nitric oxide (NO) in the regulation of uterine contractility has yet to be clearly defined. We evaluated the effect of NO (in the form of S-nitroso-cysteine, CysNO) upon uterine contractility and guanosine 3',5'-cyclic monophosphate (cyclic GMP) accumulation in pregnant and nonpregnant guinea-pig myometrium. 2. While CysNO had no effect upon spontaneous contractile activity in either pregnant or nonpregnant uterine tissues, addition of CysNO resulted in an immediate and reversible relaxation of oxytocin- or acetylcholine (ACh)-evoked contractions. 3. Relaxation of agonist-evoked contractions in response to CysNO was associated with significant elevations in intracellular cyclic GMP concentrations ([cyclic GMP]i). 4. Elevations in [cyclic GMP]i were not required for relaxation, as inhibition of guanylyl cyclase by methylene blue prevented [cyclic GMP]i accumulation while having no effect upon the ability of CysNO to relax agonist-evoked contractions. 5. Addition of the cyclic GMP-analogues, 8-Br-cyclic GMP and PET-cyclic GMP, only at high concentrations, produced partial relaxation of agonist-contracted tissues, suggesting the possibility that cyclic GMP may be sufficient but not necessary for myometrial relaxation. 6. Our studies not only provide evidence for a functional role for NO-modulation of agonist-evoked contractions in the pregnant and nonpregnant guinea-pig uterus, but also that these occur by a mechanism which is not dependent upon guanylyl cyclase activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma H., Ishikawa M., Sekizaki S. Endothelium-dependent inhibition of platelet aggregation. Br J Pharmacol. 1986 Jun;88(2):411–415. doi: 10.1111/j.1476-5381.1986.tb10218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialecki R. A., Stinson-Fisher C. KCa channel antagonists reduce NO donor-mediated relaxation of vascular and tracheal smooth muscle. Am J Physiol. 1995 Jan;268(1 Pt 1):L152–L159. doi: 10.1152/ajplung.1995.268.1.L152. [DOI] [PubMed] [Google Scholar]
- Bolotina V. M., Najibi S., Palacino J. J., Pagano P. J., Cohen R. A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994 Apr 28;368(6474):850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- Buhimschi I., Yallampalli C., Dong Y. L., Garfield R. E. Involvement of a nitric oxide-cyclic guanosine monophosphate pathway in control of human uterine contractility during pregnancy. Am J Obstet Gynecol. 1995 May;172(5):1577–1584. doi: 10.1016/0002-9378(95)90500-6. [DOI] [PubMed] [Google Scholar]
- Conrad K. P., Joffe G. M., Kruszyna H., Kruszyna R., Rochelle L. G., Smith R. P., Chavez J. E., Mosher M. D. Identification of increased nitric oxide biosynthesis during pregnancy in rats. FASEB J. 1993 Apr 1;7(6):566–571. [PubMed] [Google Scholar]
- Diamond J. Lack of correlation between cyclic GMP elevation and relaxation of nonvascular smooth muscle by nitroglycerin, nitroprusside, hydroxylamine and sodium azide. J Pharmacol Exp Ther. 1983 May;225(2):422–426. [PubMed] [Google Scholar]
- Franchi A. M., Chaud M., Rettori V., Suburo A., McCann S. M., Gimeno M. Role of nitric oxide in eicosanoid synthesis and uterine motility in estrogen-treated rat uteri. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):539–543. doi: 10.1073/pnas.91.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Nitric oxide decreases cytosolic free calcium in Balb/c 3T3 fibroblasts by a cyclic GMP-independent mechanism. J Biol Chem. 1991 Jan 5;266(1):9–12. [PubMed] [Google Scholar]
- Gibson A., Babbedge R., Brave S. R., Hart S. L., Hobbs A. J., Tucker J. F., Wallace P., Moore P. K. An investigation of some S-nitrosothiols, and of hydroxy-arginine, on the mouse anococcygeus. Br J Pharmacol. 1992 Nov;107(3):715–721. doi: 10.1111/j.1476-5381.1992.tb14512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi H., Yallampalli C., Garfield R. E. Gestational changes in L-arginine-induced relaxation of pregnant rat and human myometrial smooth muscle. Am J Obstet Gynecol. 1993 Nov;169(5):1327–1337. doi: 10.1016/0002-9378(93)90301-x. [DOI] [PubMed] [Google Scholar]
- Kannan M. S., Johnson D. E. Modulation of nitric oxide-dependent relaxation of pig tracheal smooth muscle by inhibitors of guanylyl cyclase and calcium activated potassium channels. Life Sci. 1995;56(25):2229–2238. doi: 10.1016/0024-3205(95)00212-o. [DOI] [PubMed] [Google Scholar]
- Kannan M. S., Johnson D. E. Nitric oxide mediates the neural nonadrenergic, noncholinergic relaxation of pig tracheal smooth muscle. Am J Physiol. 1992 Apr;262(4 Pt 1):L511–L514. doi: 10.1152/ajplung.1992.262.4.L511. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Moritoki H., Takei M., Kasai T., Matsumura Y., Ishida Y. Possible involvement of prostaglandins in the action of ATP on guinea-pig uterus. J Pharmacol Exp Ther. 1979 Oct;211(1):104–111. [PubMed] [Google Scholar]
- Natuzzi E. S., Ursell P. C., Harrison M., Buscher C., Riemer R. K. Nitric oxide synthase activity in the pregnant uterus decreases at parturition. Biochem Biophys Res Commun. 1993 Jul 15;194(1):1–8. doi: 10.1006/bbrc.1993.1776. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Papka R. E., McNeill D. L. Distribution of NADPH-diaphorase-positive nerves in the uterine cervix and neurons in dorsal root and paracervical ganglia of the female rat. Neurosci Lett. 1992 Dec 7;147(2):224–228. doi: 10.1016/0304-3940(92)90601-3. [DOI] [PubMed] [Google Scholar]
- Schiemann W. P., Buxton I. L. Adenosine A1-receptor coupling to phosphoinositide metabolism in pregnant guinea pig myometrium. Am J Physiol. 1991 Nov;261(5 Pt 1):E665–E672. doi: 10.1152/ajpendo.1991.261.5.E665. [DOI] [PubMed] [Google Scholar]
- Sekhar K. R., Hatchett R. J., Shabb J. B., Wolfe L., Francis S. H., Wells J. N., Jastorff B., Butt E., Chakinala M. M., Corbin J. D. Relaxation of pig coronary arteries by new and potent cGMP analogs that selectively activate type I alpha, compared with type I beta, cGMP-dependent protein kinase. Mol Pharmacol. 1992 Jul;42(1):103–108. [PubMed] [Google Scholar]
- Shew R. L., Papka R. E., McNeill D. L., Yee J. A. NADPH-diaphorase-positive nerves and the role of nitric oxide in CGRP relaxation of uterine contraction. Peptides. 1993 May-Jun;14(3):637–641. doi: 10.1016/0196-9781(93)90157-c. [DOI] [PubMed] [Google Scholar]
- Sladek S. M., Regenstein A. C., Lykins D., Roberts J. M. Nitric oxide synthase activity in pregnant rabbit uterus decreases on the last day of pregnancy. Am J Obstet Gynecol. 1993 Nov;169(5):1285–1291. doi: 10.1016/0002-9378(93)90295-t. [DOI] [PubMed] [Google Scholar]
- Smith M. A., Buxton I. L., Westfall D. P. Pharmacological classification of receptors for adenyl purines in guinea pig myometrium. J Pharmacol Exp Ther. 1988 Dec;247(3):1059–1063. [PubMed] [Google Scholar]
- Smith M. A., Silverstein J. L., Westfall D. P., Buxton I. L. Dissociation between adenosine receptors and adenylate cyclase in the smooth muscle of guinea pig myometrium. Cell Signal. 1989;1(4):357–365. doi: 10.1016/0898-6568(89)90054-5. [DOI] [PubMed] [Google Scholar]
- Szabó C., Zingarelli B., Salzman A. L. Role of poly-ADP ribosyltransferase activation in the vascular contractile and energetic failure elicited by exogenous and endogenous nitric oxide and peroxynitrite. Circ Res. 1996 Jun;78(6):1051–1063. doi: 10.1161/01.res.78.6.1051. [DOI] [PubMed] [Google Scholar]
- Telfer J. F., Lyall F., Norman J. E., Cameron I. T. Identification of nitric oxide synthase in human uterus. Hum Reprod. 1995 Jan;10(1):19–23. doi: 10.1093/humrep/10.1.19. [DOI] [PubMed] [Google Scholar]
- Thornbury K. D., Hollywood M. A., McHale N. G. Mediation by nitric oxide of neurogenic relaxation of the urinary bladder neck muscle in sheep. J Physiol. 1992;451:133–144. doi: 10.1113/jphysiol.1992.sp019157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S. M., Dalziel H. H., Bradley M. E., Buxton I. L., Keef K., Westfall D. P., Sanders K. M. Involvement of cyclic GMP in non-adrenergic, non-cholinergic inhibitory neurotransmission in dog proximal colon. Br J Pharmacol. 1992 Dec;107(4):1075–1082. doi: 10.1111/j.1476-5381.1992.tb13409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner C. P., Lizasoain I., Baylis S. A., Knowles R. G., Charles I. G., Moncada S. Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5212–5216. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Word R. A., Casey M. L., Kamm K. E., Stull J. T. Effects of cGMP on [Ca2+]i, myosin light chain phosphorylation, and contraction in human myometrium. Am J Physiol. 1991 Apr;260(4 Pt 1):C861–C867. doi: 10.1152/ajpcell.1991.260.4.C861. [DOI] [PubMed] [Google Scholar]
- Yallampalli C., Garfield R. E., Byam-Smith M. Nitric oxide inhibits uterine contractility during pregnancy but not during delivery. Endocrinology. 1993 Oct;133(4):1899–1902. doi: 10.1210/endo.133.4.8404632. [DOI] [PubMed] [Google Scholar]
- Yallampalli C., Izumi H., Byam-Smith M., Garfield R. E. An L-arginine-nitric oxide-cyclic guanosine monophosphate system exists in the uterus and inhibits contractility during pregnancy. Am J Obstet Gynecol. 1994 Jan;170(1 Pt 1):175–185. doi: 10.1016/s0002-9378(94)70405-8. [DOI] [PubMed] [Google Scholar]


