Abstract
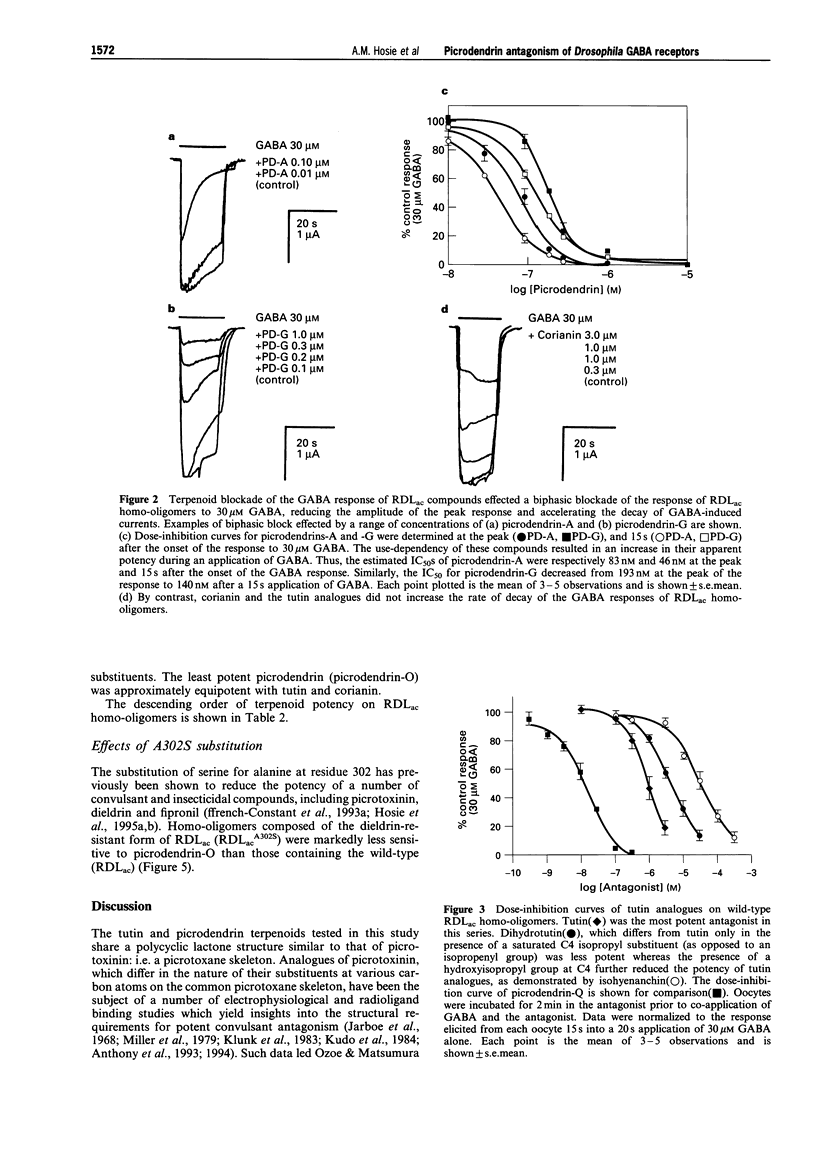
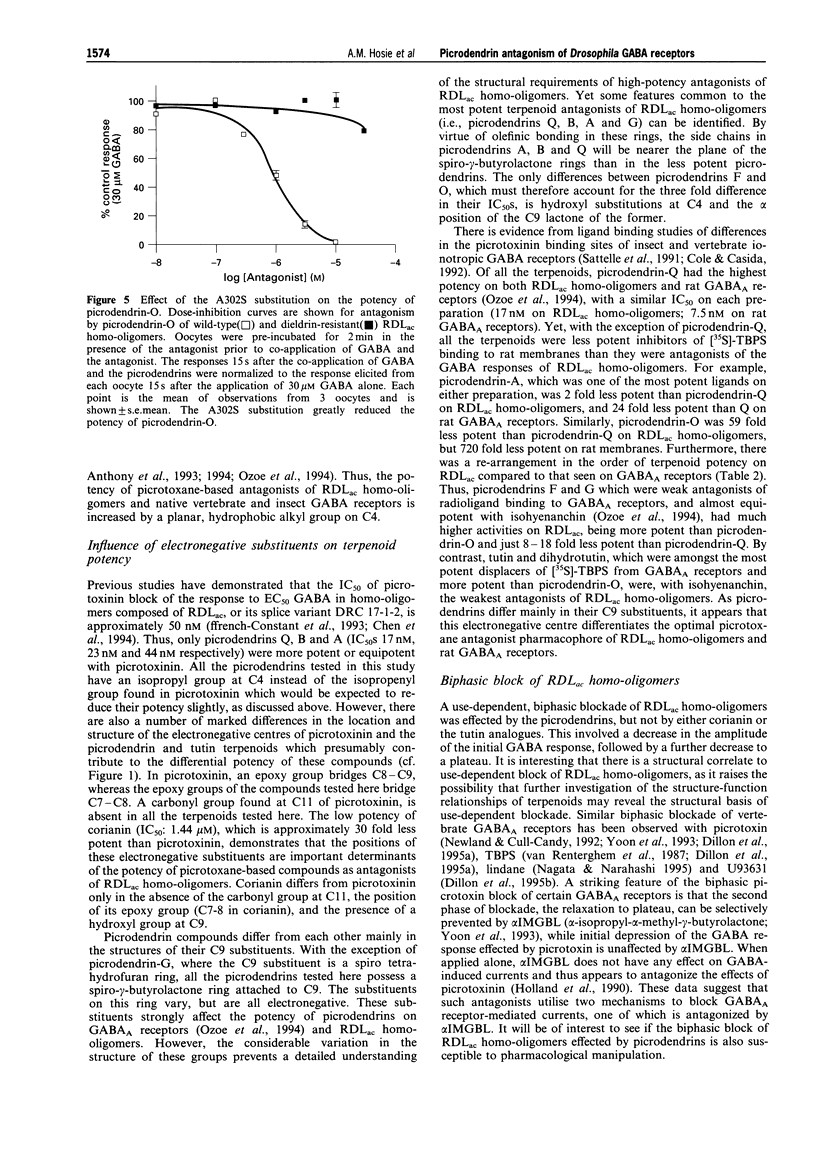
1. A series of terpenoid compounds, recently isolated from Picrodendron baccatum, share a picrotoxane skeleton with picrotoxinin, an antagonist of ionotropic GABA receptors. Referred to as picrodendrins, they inhibit the binding of [35S]-tert-butylbicyclophosphorothionate (TBPS) to rat GABAA receptors. Hitherto, their effects on GABA receptors have not been investigated electrophysiologically. Under two-electrode voltage-clamp, the actions of picrodendrins and related terpenoids have been assayed on homooligomeric GABA receptors formed by the expression of a Drosophila GABA receptor subunit (RDLac) in Xenopus oocytes. 2. All the terpenoids tested, dose-dependently antagonized currents induced by 30 microM (EC50) GABA. 3. Tutin and its analogues (dihydrotutin and isohyenanchin) differ in the structure of their axial C4 substituents. Of these compounds, tutin, which bears an isopropenyl group at this carbon atom, was the most potent antagonist of RDLac homo-oligomers, whereas isohyenanchin, which bears a hydroxyisopropyl group, was the least potent antagonist tested. 4. Picrodendrins differ mainly in the structure of their C9 substituents. The IC50s of picrodendrins ranged from 17 +/- 1.3 nM (picrodendrin-Q) to 1006 +/- 1.3 nM (picrodendrin-O). As such, the most potent picrodendrins (Q, A and B) were approximately equipotent with picrotoxinin as antagonists of RDLac homo-oligomers. 5. Certain picrodendrin compounds effected a use-dependent blockade of RDLac homo-oligomers. Such a biphasic block was not observed with tutin analogues. 6. Picrotoxin-resistant RDLacA3025 homo-oligomers, which have a single amino acid substitution (A302S) in the 2nd transmembrane region, were markedly less sensitive to picrodendrin-O than the wild-type, dieldrin-sensitive, homo-oligomers. 7. The relative potency of tutin analogues demonstrates that the structure-activity relationship of the C4 substituent of picrotoxane-based compounds is conserved in vertebrates and insects. However, the relative order of potency of picrodendrins on RDLac homo-oligomers is distinctly different from that observed in previous radioligand binding studies performed on vertebrate GABAA receptors. As picrodendrin compounds differ in the structure of their C9 substituents, these data suggest that the optimal convulsant pharmacophores of vertebrate GABAA receptors and RDLac homo-oligomers differ with respect to this substituent.
Full text
PDF

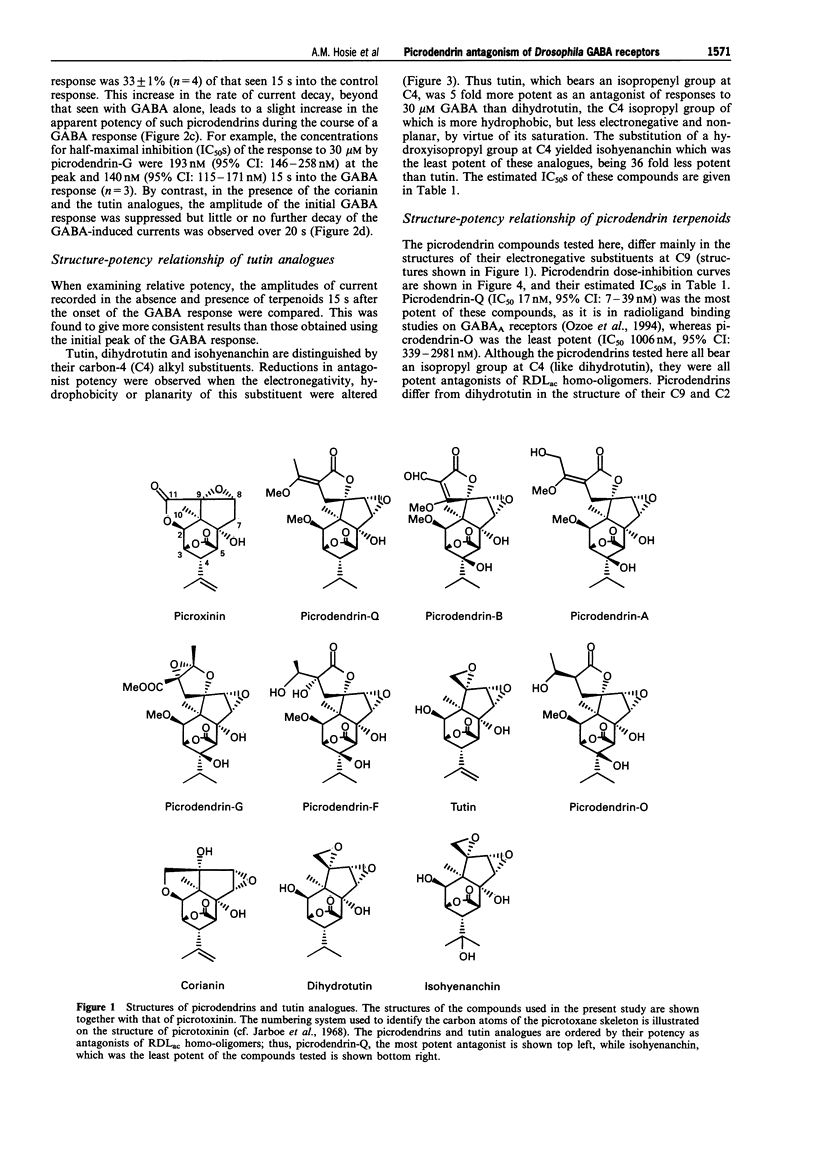
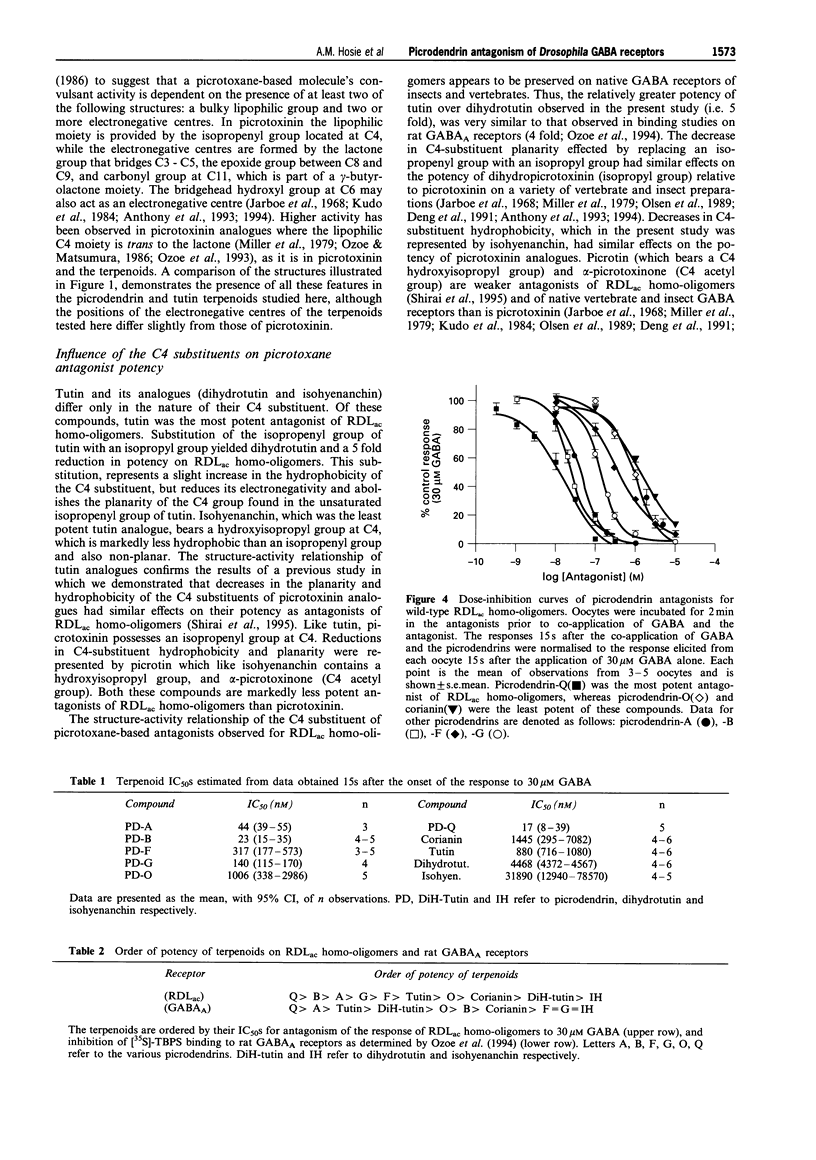


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony N. M., Holyoke C. W., Jr, Sattelle D. B. Blocking actions of picrotoxinin analogues on insect (Periplaneta americana) GABA receptors. Neurosci Lett. 1994 Apr 25;171(1-2):67–69. doi: 10.1016/0304-3940(94)90606-8. [DOI] [PubMed] [Google Scholar]
- Aronstein K., Ffrench-Constant R. Immunocytochemistry of a novel GABA receptor subunit Rdl in Drosophila melanogaster. Invert Neurosci. 1995;1(1):25–31. doi: 10.1007/BF02331829. [DOI] [PubMed] [Google Scholar]
- Bloomquist J. R. Cyclodiene resistance at the insect GABA receptor/chloride channel complex confers broad cross resistance to convulsants and experimental phenylpyrazole insecticides. Arch Insect Biochem Physiol. 1994;26(1):69–79. doi: 10.1002/arch.940260106. [DOI] [PubMed] [Google Scholar]
- Buckingham S. D., Hosie A. M., Roush R. L., Sattelle D. B. Actions of agonists and convulsant antagonists on a Drosophila melanogaster GABA receptor (Rdl) homo-oligomer expressed in Xenopus oocytes. Neurosci Lett. 1994 Nov 7;181(1-2):137–140. doi: 10.1016/0304-3940(94)90578-9. [DOI] [PubMed] [Google Scholar]
- Chen R., Belelli D., Lambert J. J., Peters J. A., Reyes A., Lan N. C. Cloning and functional expression of a Drosophila gamma-aminobutyric acid receptor. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6069–6073. doi: 10.1073/pnas.91.13.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole L. M., Roush R. T., Casida J. E. Drosophila GABA-gated chloride channel: modified [3H]EBOB binding site associated with Ala-->Ser or Gly mutants of Rdl subunit. Life Sci. 1995;56(10):757–765. doi: 10.1016/0024-3205(95)00006-r. [DOI] [PubMed] [Google Scholar]
- Dillon G. H., Im W. B., Carter D. B., McKinley D. D. Enhancement by GABA of the association rate of picrotoxin and tert-butylbicyclophosphorothionate to the rat cloned alpha 1 beta 2 gamma 2 GABAA receptor subtype. Br J Pharmacol. 1995 Jun;115(3):539–545. doi: 10.1111/j.1476-5381.1995.tb16368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon G. H., Im W. B., Pregenzer J. F., Carter D. B., Hamilton B. J. [4-Dimethyl-3-t-butylcarboxyl-4,5-dihydro (1,5-a) quinoxaline] is a novel ligand to the picrotoxin site on GABAA receptors, and decreases single-channel open probability. J Pharmacol Exp Ther. 1995 Feb;272(2):597–603. [PubMed] [Google Scholar]
- Ffrench-Constant R. H., Mortlock D. P., Shaffer C. D., MacIntyre R. J., Roush R. T. Molecular cloning and transformation of cyclodiene resistance in Drosophila: an invertebrate gamma-aminobutyric acid subtype A receptor locus. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7209–7213. doi: 10.1073/pnas.88.16.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ffrench-Constant R. H., Rocheleau T. A., Steichen J. C., Chalmers A. E. A point mutation in a Drosophila GABA receptor confers insecticide resistance. Nature. 1993 Jun 3;363(6428):449–451. doi: 10.1038/363449a0. [DOI] [PubMed] [Google Scholar]
- Harrison J. B., Chen H. H., Sattelle E., Barker P. J., Huskisson N. S., Rauh J. J., Bai D., Sattelle D. B. Immunocytochemical mapping of a C-terminus anti-peptide antibody to the GABA receptor subunit, RDL in the nervous system in Drosophila melanogaster. Cell Tissue Res. 1996 May;284(2):269–278. doi: 10.1007/s004410050587. [DOI] [PubMed] [Google Scholar]
- Holland K. D., Ferrendelli J. A., Covey D. F., Rothman S. M. Physiological regulation of the picrotoxin receptor by gamma-butyrolactones and gamma-thiobutyrolactones in cultured hippocampal neurons. J Neurosci. 1990 Jun;10(6):1719–1727. doi: 10.1523/JNEUROSCI.10-06-01719.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie A. M., Baylis H. A., Buckingham S. D., Sattelle D. B. Actions of the insecticide fipronil, on dieldrin-sensitive and- resistant GABA receptors of Drosophila melanogaster. Br J Pharmacol. 1995 Jul;115(6):909–912. doi: 10.1111/j.1476-5381.1995.tb15896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie A. M., Sattelle D. B. Allosteric modulation of an expressed homo-oligomeric GABA-gated chloride channel of Drosophila melanogaster. Br J Pharmacol. 1996 Mar;117(6):1229–1237. doi: 10.1111/j.1476-5381.1996.tb16720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie A. M., Shirai Y., Buckingham S. D., Rauh J. J., Roush R. T., Baylis H. A., Sattelle D. B. Blocking actions of BIDN, a bicyclic dinitrile convulsant compound, on wild-type and dieldrin-resistant GABA receptor homo-oligomers of Drosophila melanogaster expressed in Xenopus oocytes. Brain Res. 1995 Sep 25;693(1-2):257–260. doi: 10.1016/0006-8993(95)00605-p. [DOI] [PubMed] [Google Scholar]
- Jarboe C. H., Poerter L. A., Buckler R. T. Structural aspects of picrotoxinin action. J Med Chem. 1968 Jul;11(4):729–731. doi: 10.1021/jm00310a020. [DOI] [PubMed] [Google Scholar]
- Kaku K., Matsumura F. Identification of the site of mutation within the M2 region of the GABA receptor of the cyclodiene-resistant German cockroach. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1994 Jul;108(3):367–376. [PubMed] [Google Scholar]
- Klunk W. E., Kalman B. L., Ferrendelli J. A., Covey D. F. Computer-assisted modeling of the picrotoxinin and gamma-butyrolactone receptor site. Mol Pharmacol. 1983 Mar;23(2):511–518. [PubMed] [Google Scholar]
- Kudo Y., Niwa H., Tanaka A., Yamada K. Actions of picrotoxinin and related compounds on the frog spinal cord: the role of a hydroxyl-group at the 6-position in antagonizing the actions of amino acids and presynaptic inhibition. Br J Pharmacol. 1984 Feb;81(2):373–380. doi: 10.1111/j.1476-5381.1984.tb10088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. J., Rocheleau T., Zhang H. G., Jackson M. B., ffrench-Constant R. H. Expression of a Drosophila GABA receptor in a baculovirus insect cell system. Functional expression of insecticide susceptible and resistant GABA receptors from the cyclodiene resistance gene Rdl. FEBS Lett. 1993 Dec 13;335(3):315–318. doi: 10.1016/0014-5793(93)80409-n. [DOI] [PubMed] [Google Scholar]
- Lummis S. C., Buckingham S. D., Rauh J. J., Sattelle D. B. Blocking actions of heptachlor at an insect central nervous system GABA receptor. Proc R Soc Lond B Biol Sci. 1990 May 22;240(1297):97–106. doi: 10.1098/rspb.1990.0029. [DOI] [PubMed] [Google Scholar]
- Lummis S. C., Sattelle D. B. Insect central nervous system gamma-aminobutyric acid. Neurosci Lett. 1985 Sep 16;60(1):13–18. doi: 10.1016/0304-3940(85)90374-x. [DOI] [PubMed] [Google Scholar]
- Matsumura F., Ghiasuddin S. M. Evidence for similarities between cyclodiene type insecticides and picrotoxinin in their action mechanisms. J Environ Sci Health B. 1983;18(1):1–14. doi: 10.1080/03601238309372355. [DOI] [PubMed] [Google Scholar]
- Millar N. S., Buckingham S. D., Sattelle D. B. Stable expression of a functional homo-oligomeric Drosophila GABA receptor in a Drosophila cell line. Proc Biol Sci. 1994 Dec 22;258(1353):307–314. doi: 10.1098/rspb.1994.0178. [DOI] [PubMed] [Google Scholar]
- Miyazaki M., Matsumura F., Beeman R. W. DNA sequence and site of mutation of the GABA receptor of cyclodiene-resistant red flour beetle, Tribolium castaneum. Comp Biochem Physiol B Biochem Mol Biol. 1995 Jul;111(3):399–406. doi: 10.1016/0305-0491(95)00007-u. [DOI] [PubMed] [Google Scholar]
- Nagata K., Narahashi T. Differential effects of hexachlorocyclohexane isomers on the GABA receptor-chloride channel complex in rat dorsal root ganglion neurons. Brain Res. 1995 Dec 15;704(1):85–91. doi: 10.1016/0006-8993(95)01108-0. [DOI] [PubMed] [Google Scholar]
- Newland C. F., Cull-Candy S. G. On the mechanism of action of picrotoxin on GABA receptor channels in dissociated sympathetic neurones of the rat. J Physiol. 1992 Feb;447:191–213. doi: 10.1113/jphysiol.1992.sp018998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. W., Szamraj O., Miller T. t-[35S]butylbicyclophosphorothionate binding sites in invertebrate tissues. J Neurochem. 1989 Apr;52(4):1311–1318. doi: 10.1111/j.1471-4159.1989.tb01880.x. [DOI] [PubMed] [Google Scholar]
- Sattelle D. B., Lummis S. C., Wong J. F., Rauh J. J. Pharmacology of insect GABA receptors. Neurochem Res. 1991 Mar;16(3):363–374. doi: 10.1007/BF00966100. [DOI] [PubMed] [Google Scholar]
- Shirai Y., Hosie A. M., Buckingham S. D., Holyoke C. W., Jr, Baylis H. A., Sattelle D. B. Actions of picrotoxinin analogues on an expressed, homo-oligomeric GABA receptor of Drosophila melanogaster. Neurosci Lett. 1995 Apr 7;189(1):1–4. doi: 10.1016/0304-3940(95)11432-v. [DOI] [PubMed] [Google Scholar]
- Thompson M., Shotkoski F., ffrench-Constant R. Cloning and sequencing of the cyclodiene insecticide resistance gene from the yellow fever mosquito Aedes aegypti. Conservation of the gene and resistance associated mutation with Drosophila. FEBS Lett. 1993 Jul 5;325(3):187–190. doi: 10.1016/0014-5793(93)81070-g. [DOI] [PubMed] [Google Scholar]
- Thompson M., Steichen J. C., ffrench-Constant R. H. Conservation of cyclodiene insecticide resistance-associated mutations in insects. Insect Mol Biol. 1993;2(3):149–154. doi: 10.1111/j.1365-2583.1993.tb00134.x. [DOI] [PubMed] [Google Scholar]
- Twyman R. E., Rogers C. J., Macdonald R. L. Pentobarbital and picrotoxin have reciprocal actions on single GABAA receptor channels. Neurosci Lett. 1989 Jan 2;96(1):89–95. doi: 10.1016/0304-3940(89)90248-6. [DOI] [PubMed] [Google Scholar]
- Van Renterghem C., Bilbe G., Moss S., Smart T. G., Constanti A., Brown D. A., Barnard E. A. GABA receptors induced in Xenopus oocytes by chick brain mRNA: evaluation of TBPS as a use-dependent channel-blocker. Brain Res. 1987 Apr;388(1):21–31. doi: 10.1016/0169-328x(87)90017-9. [DOI] [PubMed] [Google Scholar]
- Wafford K. A., Lummis S. C., Sattelle D. B. Block of an insect central nervous system GABA receptor by cyclodiene and cyclohexane insecticides. Proc R Soc Lond B Biol Sci. 1989 Jun 22;237(1286):53–61. doi: 10.1098/rspb.1989.0036. [DOI] [PubMed] [Google Scholar]
- Yoon K. W., Covey D. F., Rothman S. M. Multiple mechanisms of picrotoxin block of GABA-induced currents in rat hippocampal neurons. J Physiol. 1993 May;464:423–439. doi: 10.1113/jphysiol.1993.sp019643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. G., ffrench-Constant R. H., Jackson M. B. A unique amino acid of the Drosophila GABA receptor with influence on drug sensitivity by two mechanisms. J Physiol. 1994 Aug 15;479(Pt 1):65–75. doi: 10.1113/jphysiol.1994.sp020278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ffrench-Constant R. H., Rocheleau T. A. Drosophila gamma-aminobutyric acid receptor gene Rdl shows extensive alternative splicing. J Neurochem. 1993 Jun;60(6):2323–2326. doi: 10.1111/j.1471-4159.1993.tb03523.x. [DOI] [PubMed] [Google Scholar]
- ffrench-Constant R. H., Steichen J. C., Rocheleau T. A., Aronstein K., Roush R. T. A single-amino acid substitution in a gamma-aminobutyric acid subtype A receptor locus is associated with cyclodiene insecticide resistance in Drosophila populations. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1957–1961. doi: 10.1073/pnas.90.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]


