Abstract
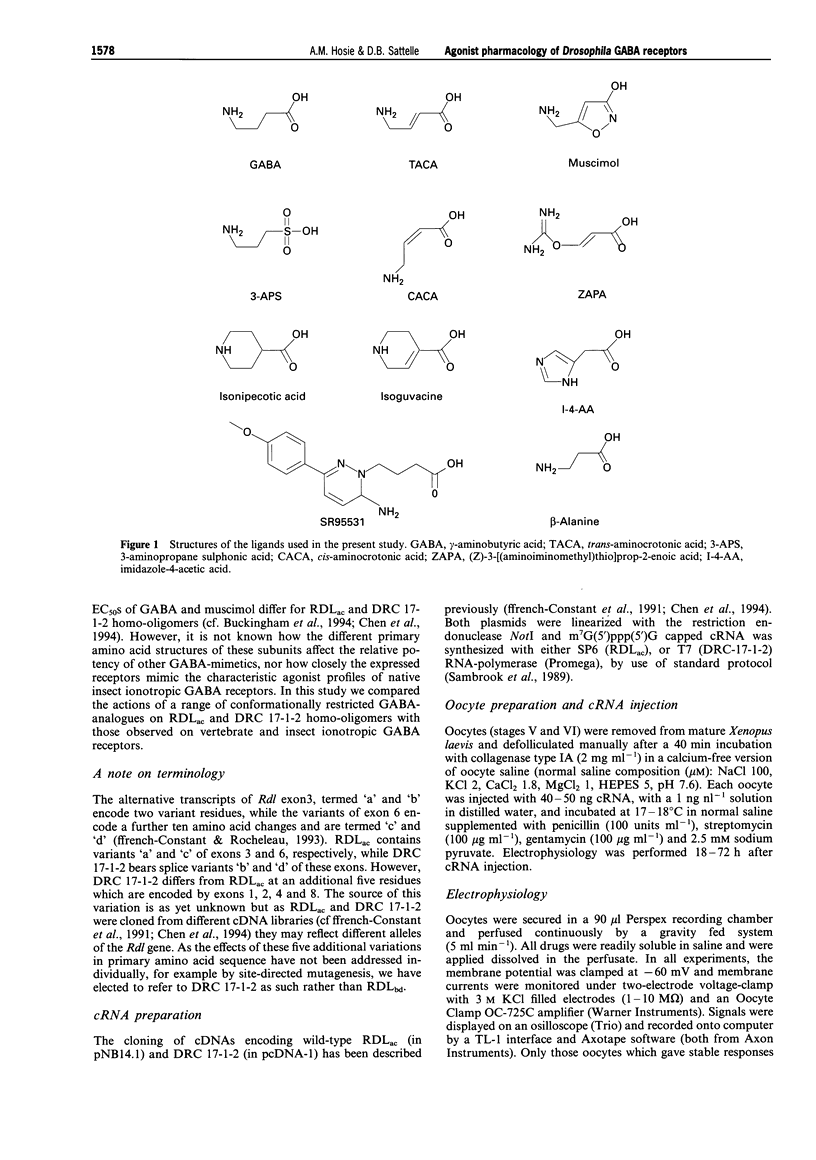
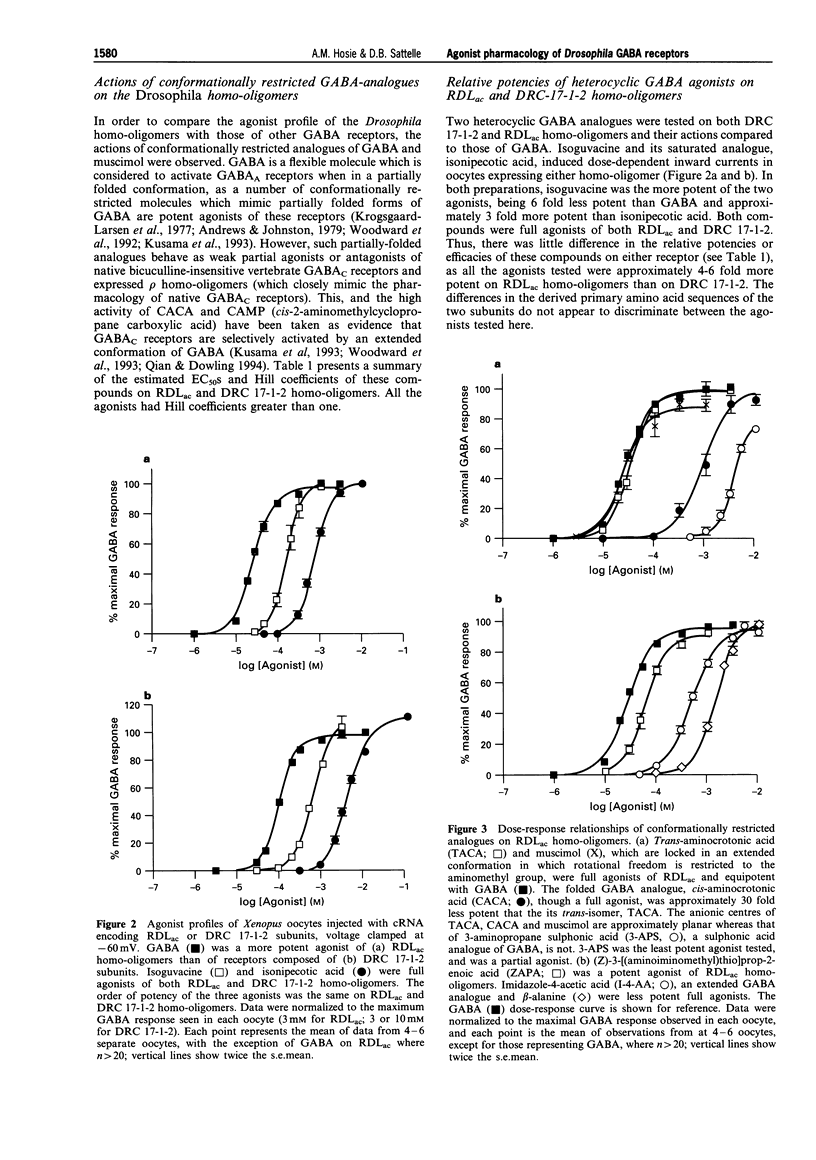
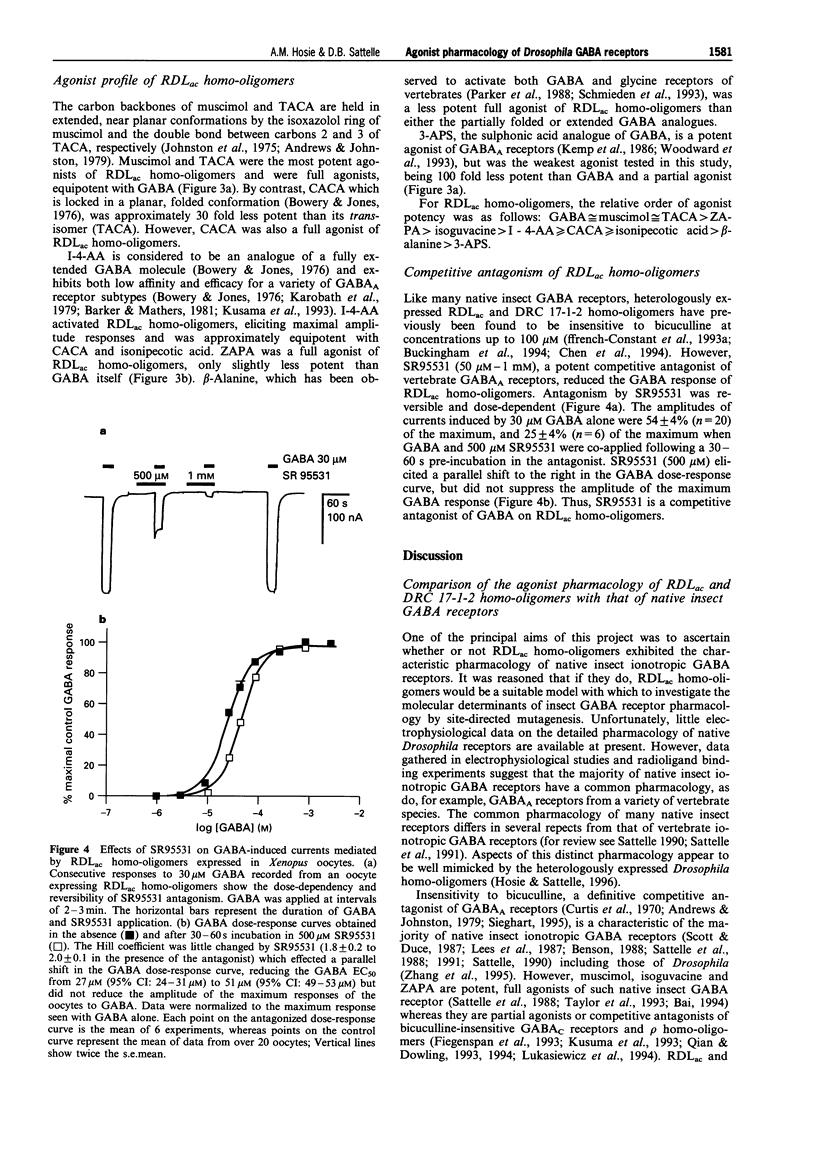
1. The Drosophila melanogaster gamma-aminobutyric acid (GABA) receptor subunits, RDLac and DRC 17-1-2, form functional homo-oligomeric receptors when heterologously expressed in Xenopus laevis oocytes. The subunits differ in only 17 amino acids, principally in regions of the N-terminal domain which determine agonist pharmacology in vertebrate ionotropic neurotransmitter receptors. A range of conformationally restricted GABA analogues were tested on the two homo-oligomers and their agonists pharmacology compared with that of insect and vertebrate iontropic GABA receptors. 2. The actions of GABA, isoguvacine and isonipecotic acid on RDLac and DRC 17-1-2 homo-oligomers were compared, by use of two-electrode voltage-clamp. All three compounds were full agonists of both receptors, but were 4-6 fold less potent agonists of DRC 17-1-2 homo-oligomers than of RDLac. However, the relative potencies of these agonists on each receptor were very similar. 3. A more complete agonist profile was established for RDLac homo-oligomers. The most potent agonists of these receptors were GABA, muscimol and trans-aminocrotonic acid (TACA), which were approximately equipotent. RDLac homo-oligomers were fully activated by a range of GABA analogues, with the order of potency: GABA > ZAPA ((Z)-3-[(aminoiminomethyl)thio]prop-2-enoic acid) > isoguvacine > imidazole-4-acetic acid > or = isonipecotic acid > or = cis-aminocrotonic acid (CACA) > beta-alanine. 3-Aminopropane sulphonic acid (3-APS), a partial agonist of RDLac homo-oligomers, was the weakest agonist tested and 100 fold less potent than GABA. 4. SR95531, an antagonist of vertebrate GABAA receptors, competitively inhibited the GABA responses of RDLac homo-oligomers, which have previously been found to insensitive to bicuculline. However, its potency (IC50 500 microM) was much reduced when compared to GABAA receptors. 5. The agonist pharmacology of Drosophila RDLac homo-oligomers exhibits aspects of the characteristic pharmacology of certain native insect GABA receptors which distinguish them from vertebrate GABA receptors. The high potency and efficacy of isoguvacine and ZAPA distinguishes RDLac homo-oligomers from bicuculline-insensitive vertebrate GABAC receptors, while the low potency of SR95531 and 3-APS distinguishes them from GABAA receptors. The differences in the potency of agonists on RDLac and DRC 17-1-2 homo-oligomers observed in the present study may assist in identification of further molecular determinants of GABA receptor function.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan R. D., Dickenson H. W., Hiern B. P., Johnston G. A., Kazlauskas R. Isothiouronium compounds as gamma-aminobutyric acid agonists. Br J Pharmacol. 1986 Jun;88(2):379–387. doi: 10.1111/j.1476-5381.1986.tb10214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin J., Weiss D. S. GABAA receptor needs two homologous domains of the beta-subunit for activation by GABA but not by pentobarbital. Nature. 1993 Dec 9;366(6455):565–569. doi: 10.1038/366565a0. [DOI] [PubMed] [Google Scholar]
- Amin J., Weiss D. S. Homomeric rho 1 GABA channels: activation properties and domains. Receptors Channels. 1994;2(3):227–236. [PubMed] [Google Scholar]
- Andrews P. R., Johnston G. A. GABA agonists and antagonists. Biochem Pharmacol. 1979 Sep 15;28(18):2697–2702. doi: 10.1016/0006-2952(79)90549-5. [DOI] [PubMed] [Google Scholar]
- Aprison M. H., Galvez-Ruano E., Lipkowitz K. B. Identification of a second glycine-like fragment on the strychnine molecule. J Neurosci Res. 1995 Feb 15;40(3):396–400. doi: 10.1002/jnr.490400314. [DOI] [PubMed] [Google Scholar]
- Aronstein K., Ffrench-Constant R. Immunocytochemistry of a novel GABA receptor subunit Rdl in Drosophila melanogaster. Invert Neurosci. 1995;1(1):25–31. doi: 10.1007/BF02331829. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Mathers D. A. GABA analogues activate channels of different duration on cultured mouse spinal neurons. Science. 1981 Apr 17;212(4492):358–361. doi: 10.1126/science.6259733. [DOI] [PubMed] [Google Scholar]
- Benson J. A. Bicuculline blocks the response to acetylcholine and nicotine but not to muscarine or GABA in isolated insect neuronal somata. Brain Res. 1988 Aug 16;458(1):65–71. doi: 10.1016/0006-8993(88)90496-9. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Jones G. P. A comparison of gamma-aminobutyric acid and the semi-rigid analogues 4-aminotetrolic acid, 4-aminocrotonic acid and imidazole-4-acetic acid on the isolated superior cervical ganglion of the rat. Br J Pharmacol. 1976 Mar;56(3):323–330. doi: 10.1111/j.1476-5381.1976.tb07646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham S. D., Hosie A. M., Roush R. L., Sattelle D. B. Actions of agonists and convulsant antagonists on a Drosophila melanogaster GABA receptor (Rdl) homo-oligomer expressed in Xenopus oocytes. Neurosci Lett. 1994 Nov 7;181(1-2):137–140. doi: 10.1016/0304-3940(94)90578-9. [DOI] [PubMed] [Google Scholar]
- Chen R., Belelli D., Lambert J. J., Peters J. A., Reyes A., Lan N. C. Cloning and functional expression of a Drosophila gamma-aminobutyric acid receptor. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6069–6073. doi: 10.1073/pnas.91.13.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A. GABA, bicuculline and central inhibition. Nature. 1970 Jun 27;226(5252):1222–1224. doi: 10.1038/2261222a0. [DOI] [PubMed] [Google Scholar]
- Ebert B., Wafford K. A., Whiting P. J., Krogsgaard-Larsen P., Kemp J. A. Molecular pharmacology of gamma-aminobutyric acid type A receptor agonists and partial agonists in oocytes injected with different alpha, beta, and gamma receptor subunit combinations. Mol Pharmacol. 1994 Nov;46(5):957–963. [PubMed] [Google Scholar]
- Feigenspan A., Wässle H., Bormann J. Pharmacology of GABA receptor Cl- channels in rat retinal bipolar cells. Nature. 1993 Jan 14;361(6408):159–162. doi: 10.1038/361159a0. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant R. H., Mortlock D. P., Shaffer C. D., MacIntyre R. J., Roush R. T. Molecular cloning and transformation of cyclodiene resistance in Drosophila: an invertebrate gamma-aminobutyric acid subtype A receptor locus. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7209–7213. doi: 10.1073/pnas.88.16.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ffrench-Constant R. H., Rocheleau T. A., Steichen J. C., Chalmers A. E. A point mutation in a Drosophila GABA receptor confers insecticide resistance. Nature. 1993 Jun 3;363(6428):449–451. doi: 10.1038/363449a0. [DOI] [PubMed] [Google Scholar]
- Harrison J. B., Chen H. H., Sattelle E., Barker P. J., Huskisson N. S., Rauh J. J., Bai D., Sattelle D. B. Immunocytochemical mapping of a C-terminus anti-peptide antibody to the GABA receptor subunit, RDL in the nervous system in Drosophila melanogaster. Cell Tissue Res. 1996 May;284(2):269–278. doi: 10.1007/s004410050587. [DOI] [PubMed] [Google Scholar]
- Heaulme M., Chambon J. P., Leyris R., Molimard J. C., Wermuth C. G., Biziere K. Biochemical characterization of the interaction of three pyridazinyl-GABA derivatives with the GABAA receptor site. Brain Res. 1986 Oct 8;384(2):224–231. doi: 10.1016/0006-8993(86)91158-3. [DOI] [PubMed] [Google Scholar]
- Hosie A. M., Sattelle D. B. Allosteric modulation of an expressed homo-oligomeric GABA-gated chloride channel of Drosophila melanogaster. Br J Pharmacol. 1996 Mar;117(6):1229–1237. doi: 10.1111/j.1476-5381.1996.tb16720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. A., Curtis D. R., Beart P. M., Game C. J., McCulloch R. M., Twitchin B. Cis- and trans-4-aminocrotonic acid as GABA analogues of restricted conformation. J Neurochem. 1975 Jan;24(1):157–160. doi: 10.1111/j.1471-4159.1975.tb07642.x. [DOI] [PubMed] [Google Scholar]
- Kaku K., Matsumura F. Identification of the site of mutation within the M2 region of the GABA receptor of the cyclodiene-resistant German cockroach. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1994 Jul;108(3):367–376. [PubMed] [Google Scholar]
- Karlin A., Akabas M. H. Toward a structural basis for the function of nicotinic acetylcholine receptors and their cousins. Neuron. 1995 Dec;15(6):1231–1244. doi: 10.1016/0896-6273(95)90004-7. [DOI] [PubMed] [Google Scholar]
- Karobath M., Sperk G. Stimulation of benzodiazepine receptor binding by gamma-aminobutyric acid. Proc Natl Acad Sci U S A. 1979 Feb;76(2):1004–1006. doi: 10.1073/pnas.76.2.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp J. A., Marshall G. R., Woodruff G. N. Quantitative evaluation of the potencies of GABA-receptor agonists and antagonists using the rat hippocampal slice preparation. Br J Pharmacol. 1986 Apr;87(4):677–684. doi: 10.1111/j.1476-5381.1986.tb14585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P., Johnston G. A., Lodge D., Curtis D. R. A new class of GABA agonist. Nature. 1977 Jul 7;268(5615):53–55. doi: 10.1038/268053a0. [DOI] [PubMed] [Google Scholar]
- Kuhse J., Betz H., Kirsch J. The inhibitory glycine receptor: architecture, synaptic localization and molecular pathology of a postsynaptic ion-channel complex. Curr Opin Neurobiol. 1995 Jun;5(3):318–323. doi: 10.1016/0959-4388(95)80044-1. [DOI] [PubMed] [Google Scholar]
- Kusama T., Spivak C. E., Whiting P., Dawson V. L., Schaeffer J. C., Uhl G. R. Pharmacology of GABA rho 1 and GABA alpha/beta receptors expressed in Xenopus oocytes and COS cells. Br J Pharmacol. 1993 May;109(1):200–206. doi: 10.1111/j.1476-5381.1993.tb13554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees G., Beadle D. J., Neumann R., Benson J. A. Responses to GABA by isolated insect neuronal somata: pharmacology and modulation by a benzodiazepine and a barbiturate. Brain Res. 1987 Jan 20;401(2):267–278. doi: 10.1016/0006-8993(87)91411-9. [DOI] [PubMed] [Google Scholar]
- Levitan E. S., Schofield P. R., Burt D. R., Rhee L. M., Wisden W., Köhler M., Fujita N., Rodriguez H. F., Stephenson A., Darlison M. G. Structural and functional basis for GABAA receptor heterogeneity. Nature. 1988 Sep 1;335(6185):76–79. doi: 10.1038/335076a0. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz P. D., Maple B. R., Werblin F. S. A novel GABA receptor on bipolar cell terminals in the tiger salamander retina. J Neurosci. 1994 Mar;14(3 Pt 1):1202–1212. doi: 10.1523/JNEUROSCI.14-03-01202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M., Matsumura F., Beeman R. W. DNA sequence and site of mutation of the GABA receptor of cyclodiene-resistant red flour beetle, Tribolium castaneum. Comp Biochem Physiol B Biochem Mol Biol. 1995 Jul;111(3):399–406. doi: 10.1016/0305-0491(95)00007-u. [DOI] [PubMed] [Google Scholar]
- Mody I., De Koninck Y., Otis T. S., Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994 Dec;17(12):517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Murphy V. F., Wann K. T. The action of GABA receptor agonists and antagonists on muscle membrane conductance in Schistocerca gregaria. Br J Pharmacol. 1988 Nov;95(3):713–722. doi: 10.1111/j.1476-5381.1988.tb11697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Sumikawa K., Miledi R. Responses to GABA, glycine and beta-alanine induced in Xenopus oocytes by messenger RNA from chick and rat brain. Proc R Soc Lond B Biol Sci. 1988 Mar 22;233(1271):201–216. doi: 10.1098/rspb.1988.0019. [DOI] [PubMed] [Google Scholar]
- Polenzani L., Woodward R. M., Miledi R. Expression of mammalian gamma-aminobutyric acid receptors with distinct pharmacology in Xenopus oocytes. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4318–4322. doi: 10.1073/pnas.88.10.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H., Dowling J. E. Novel GABA responses from rod-driven retinal horizontal cells. Nature. 1993 Jan 14;361(6408):162–164. doi: 10.1038/361162a0. [DOI] [PubMed] [Google Scholar]
- Qian H., Dowling J. E. Pharmacology of novel GABA receptors found on rod horizontal cells of the white perch retina. J Neurosci. 1994 Jul;14(7):4299–4307. doi: 10.1523/JNEUROSCI.14-07-04299.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattelle D. B., Lummis S. C., Wong J. F., Rauh J. J. Pharmacology of insect GABA receptors. Neurochem Res. 1991 Mar;16(3):363–374. doi: 10.1007/BF00966100. [DOI] [PubMed] [Google Scholar]
- Sattelle D. B., Pinnock R. D., Wafford K. A., David J. A. GABA receptors on the cell-body membrane of an identified insect motor neuron. Proc R Soc Lond B Biol Sci. 1988 Jan 22;232(1269):443–456. doi: 10.1098/rspb.1988.0006. [DOI] [PubMed] [Google Scholar]
- Schmieden V., Kuhse J., Betz H. Mutation of glycine receptor subunit creates beta-alanine receptor responsive to GABA. Science. 1993 Oct 8;262(5131):256–258. doi: 10.1126/science.8211147. [DOI] [PubMed] [Google Scholar]
- Scott R. H., Duce I. R. Pharmacology of GABA receptors on skeletal muscle fibres of the locust (Schistocerca gregaria). Comp Biochem Physiol C. 1987;86(2):305–311. doi: 10.1016/0742-8413(87)90084-3. [DOI] [PubMed] [Google Scholar]
- Shirai Y., Hosie A. M., Buckingham S. D., Holyoke C. W., Jr, Baylis H. A., Sattelle D. B. Actions of picrotoxinin analogues on an expressed, homo-oligomeric GABA receptor of Drosophila melanogaster. Neurosci Lett. 1995 Apr 7;189(1):1–4. doi: 10.1016/0304-3940(95)11432-v. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995 Jun;47(2):181–234. [PubMed] [Google Scholar]
- Sigel E., Baur R., Kellenberger S., Malherbe P. Point mutations affecting antagonist affinity and agonist dependent gating of GABAA receptor channels. EMBO J. 1992 Jun;11(6):2017–2023. doi: 10.1002/j.1460-2075.1992.tb05258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. B., Olsen R. W. Functional domains of GABAA receptors. Trends Pharmacol Sci. 1995 May;16(5):162–168. doi: 10.1016/s0165-6147(00)89009-4. [DOI] [PubMed] [Google Scholar]
- Taleb O., Betz H. Expression of the human glycine receptor alpha 1 subunit in Xenopus oocytes: apparent affinities of agonists increase at high receptor density. EMBO J. 1994 Mar 15;13(6):1318–1324. doi: 10.1002/j.1460-2075.1994.tb06384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M., Shotkoski F., ffrench-Constant R. Cloning and sequencing of the cyclodiene insecticide resistance gene from the yellow fever mosquito Aedes aegypti. Conservation of the gene and resistance associated mutation with Drosophila. FEBS Lett. 1993 Jul 5;325(3):187–190. doi: 10.1016/0014-5793(93)81070-g. [DOI] [PubMed] [Google Scholar]
- Tögel M., Mossier B., Fuchs K., Sieghart W. gamma-Aminobutyric acidA receptors displaying association of gamma 3-subunits with beta 2/3 and different alpha-subunits exhibit unique pharmacological properties. J Biol Chem. 1994 Apr 29;269(17):12993–12998. [PubMed] [Google Scholar]
- Woodward R. M., Polenzani L., Miledi R. Characterization of bicuculline/baclofen-insensitive (rho-like) gamma-aminobutyric acid receptors expressed in Xenopus oocytes. II. Pharmacology of gamma-aminobutyric acidA and gamma-aminobutyric acidB receptor agonists and antagonists. Mol Pharmacol. 1993 Apr;43(4):609–625. [PubMed] [Google Scholar]
- Zhang H. G., Lee H. J., Rocheleau T., ffrench-Constant R. H., Jackson M. B. Subunit composition determines picrotoxin and bicuculline sensitivity of Drosophila gamma-aminobutyric acid receptors. Mol Pharmacol. 1995 Nov;48(5):835–840. [PubMed] [Google Scholar]
- ffrench-Constant R. H., Rocheleau T. A. Drosophila gamma-aminobutyric acid receptor gene Rdl shows extensive alternative splicing. J Neurochem. 1993 Jun;60(6):2323–2326. doi: 10.1111/j.1471-4159.1993.tb03523.x. [DOI] [PubMed] [Google Scholar]
- ffrench-Constant R. H., Steichen J. C., Rocheleau T. A., Aronstein K., Roush R. T. A single-amino acid substitution in a gamma-aminobutyric acid subtype A receptor locus is associated with cyclodiene insecticide resistance in Drosophila populations. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1957–1961. doi: 10.1073/pnas.90.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]


