Abstract
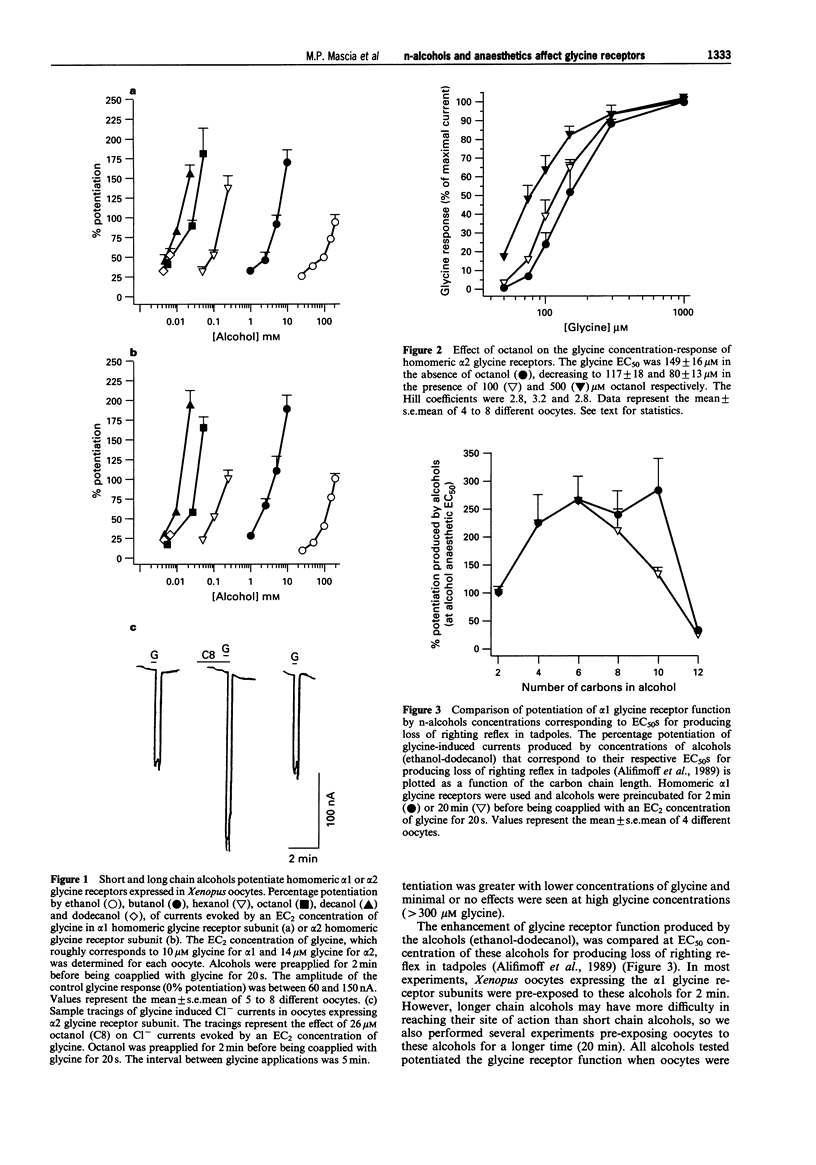
1. The effects of n-alcohols (ethanol to dodecanol) and anaesthetics on strychnine-sensitive glycine receptors were studied in Xenopus oocytes expressing homomeric alpha 1 or alpha 2 glycine receptor subunits, with the two electrode voltage-clamp recording technique. 2. The glycine-induced chloride conductance of homomeric alpha glycine receptors was potentiated by all the alcohols tested when an EC2 concentration of glycine was used. Homomeric alpha 1 and alpha 2 receptors were potentiated similarly by the n-alcohols, except that low concentrations of ethanol produced greater potentiation with alpha 1, as previously reported. 3. Increasing the n-alcohol carbon number has been shown to increase the potency of the alcohols up to decanol at concentrations corresponding to EC50s for producing loss of righting reflex in tadpoles. However, dodecanol was no more potent than decanol, and only modest potentiation (30-60%) was obtained with dodecanol, in contrast to marked (150-200%) potentiation with the other alcohols. Thus, a "cut-off' occurred at about dodecanol. 4. Propofol, alphaxalone, pentobarbitone, halothane and enflurane, reversibly potentiated the function of homomeric alpha 1 glycine receptors at concentrations which represent approximately twice the EC50 for production of anaesthesia in mammals, but ketamine and etomidate were ineffective. 5. Two novel cyclobutane compounds were tested; the anaesthetic compound (1-chloro-1,2,2-trifluorocyclobutane) from 0.5 to 5 mM potentiated the action of glycine in a concentration-dependent manner; however, the non-anaesthetic analogue (1,2-dichloro-hexfluorocyclobutane) had no effect on glycine receptor function at concentrations (25 to 80 microM) predicted to be anaesthetic, based on the lipid solubility of this compound. 6. These results suggest that the alpha subunits of strychnine-sensitive glycine receptors contain sites of action for n-alcohols, propofol, alphaxalone, pentobarbitone and volatile anaesthetics, but not for ketamine and etomidate. Potentiation of glycine receptor function may contribute to the anaesthetic action of n-alcohols and volatile agents.
Full text
PDF

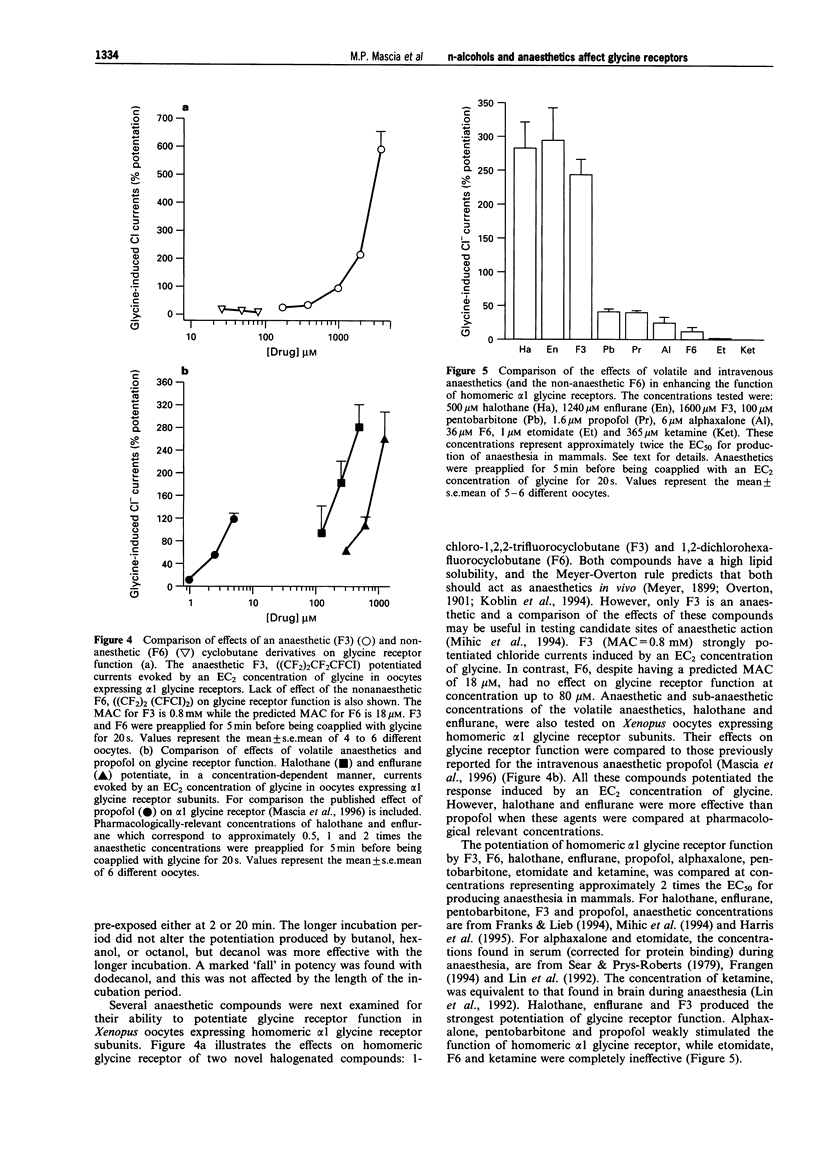


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguayo L. G., Pancetti F. C. Ethanol modulation of the gamma-aminobutyric acidA- and glycine-activated Cl- current in cultured mouse neurons. J Pharmacol Exp Ther. 1994 Jul;270(1):61–69. [PubMed] [Google Scholar]
- Alifimoff J. K., Firestone L. L., Miller K. W. Anaesthetic potencies of primary alkanols: implications for the molecular dimensions of the anaesthetic site. Br J Pharmacol. 1989 Jan;96(1):9–16. doi: 10.1111/j.1476-5381.1989.tb11777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antognini J. F., Schwartz K. Exaggerated anesthetic requirements in the preferentially anesthetized brain. Anesthesiology. 1993 Dec;79(6):1244–1249. doi: 10.1097/00000542-199312000-00015. [DOI] [PubMed] [Google Scholar]
- Betz H. Glycine receptors: heterogeneous and widespread in the mammalian brain. Trends Neurosci. 1991 Oct;14(10):458–461. doi: 10.1016/0166-2236(91)90045-v. [DOI] [PubMed] [Google Scholar]
- Béchade C., Sur C., Triller A. The inhibitory neuronal glycine receptor. Bioessays. 1994 Oct;16(10):735–744. doi: 10.1002/bies.950161008. [DOI] [PubMed] [Google Scholar]
- Celentano J. J., Gibbs T. T., Farb D. H. Ethanol potentiates GABA- and glycine-induced chloride currents in chick spinal cord neurons. Brain Res. 1988 Jul 12;455(2):377–380. doi: 10.1016/0006-8993(88)90098-4. [DOI] [PubMed] [Google Scholar]
- Collins J. G., Kendig J. J., Mason P. Anesthetic actions within the spinal cord: contributions to the state of general anesthesia. Trends Neurosci. 1995 Dec;18(12):549–553. doi: 10.1016/0166-2236(95)98377-b. [DOI] [PubMed] [Google Scholar]
- Dildy-Mayfield J. E., Mihic S. J., Liu Y., Deitrich R. A., Harris R. A. Actions of long chain alcohols on GABAA and glutamate receptors: relation to in vivo effects. Br J Pharmacol. 1996 May;118(2):378–384. doi: 10.1111/j.1476-5381.1996.tb15413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downie D. L., Hall A. C., Lieb W. R., Franks N. P. Effects of inhalational general anaesthetics on native glycine receptors in rat medullary neurones and recombinant glycine receptors in Xenopus oocytes. Br J Pharmacol. 1996 Jun;118(3):493–502. doi: 10.1111/j.1476-5381.1996.tb15430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engblom A. C., Akerman K. E. Effect of ethanol on gamma-aminobutyric acid and glycine receptor-coupled Cl- fluxes in rat brain synaptoneurosomes. J Neurochem. 1991 Aug;57(2):384–390. doi: 10.1111/j.1471-4159.1991.tb03764.x. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Mapping of general anaesthetic target sites provides a molecular basis for cutoff effects. Nature. 1985 Jul 25;316(6026):349–351. doi: 10.1038/316349a0. [DOI] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994 Feb 17;367(6464):607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Schmieden V., Schofield P. R., Seeburg P. H., Siddique T., Mohandas T. K., Becker C. M., Betz H. Alpha subunit variants of the human glycine receptor: primary structures, functional expression and chromosomal localization of the corresponding genes. EMBO J. 1990 Mar;9(3):771–776. doi: 10.1002/j.1460-2075.1990.tb08172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales T. G., Lambert J. J. The actions of propofol on inhibitory amino acid receptors of bovine adrenomedullary chromaffin cells and rodent central neurones. Br J Pharmacol. 1991 Nov;104(3):619–628. doi: 10.1111/j.1476-5381.1991.tb12479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. A., Mihic S. J., Dildy-Mayfield J. E., Machu T. K. Actions of anesthetics on ligand-gated ion channels: role of receptor subunit composition. FASEB J. 1995 Nov;9(14):1454–1462. doi: 10.1096/fasebj.9.14.7589987. [DOI] [PubMed] [Google Scholar]
- Harrison N. L., Kugler J. L., Jones M. V., Greenblatt E. P., Pritchett D. B. Positive modulation of human gamma-aminobutyric acid type A and glycine receptors by the inhalation anesthetic isoflurane. Mol Pharmacol. 1993 Sep;44(3):628–632. [PubMed] [Google Scholar]
- Hill-Venning C., Peters J. A., Lambert J. J. The interaction of steroids with inhibitory and excitatory amino acid receptors. Clin Neuropharmacol. 1992;15 (Suppl 1 Pt A):683A–684A. doi: 10.1097/00002826-199201001-00353. [DOI] [PubMed] [Google Scholar]
- Koblin D. D., Chortkoff B. S., Laster M. J., Eger E. I., 2nd, Halsey M. J., Ionescu P. Polyhalogenated and perfluorinated compounds that disobey the Meyer-Overton hypothesis. Anesth Analg. 1994 Dec;79(6):1043–1048. doi: 10.1213/00000539-199412000-00004. [DOI] [PubMed] [Google Scholar]
- Lin L. H., Chen L. L., Zirrolli J. A., Harris R. A. General anesthetics potentiate gamma-aminobutyric acid actions on gamma-aminobutyric acidA receptors expressed by Xenopus oocytes: lack of involvement of intracellular calcium. J Pharmacol Exp Ther. 1992 Nov;263(2):569–578. [PubMed] [Google Scholar]
- Malosio M. L., Marquèze-Pouey B., Kuhse J., Betz H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J. 1991 Sep;10(9):2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia M. P., Mihic S. J., Valenzuela C. F., Schofield P. R., Harris R. A. A single amino acid determines differences in ethanol actions on strychnine-sensitive glycine receptors. Mol Pharmacol. 1996 Aug;50(2):402–406. [PubMed] [Google Scholar]
- Mihic S. J., McQuilkin S. J., Eger E. I., 2nd, Ionescu P., Harris R. A. Potentiation of gamma-aminobutyric acid type A receptor-mediated chloride currents by novel halogenated compounds correlates with their abilities to induce general anesthesia. Mol Pharmacol. 1994 Nov;46(5):851–857. [PubMed] [Google Scholar]
- Pribilla I., Takagi T., Langosch D., Bormann J., Betz H. The atypical M2 segment of the beta subunit confers picrotoxinin resistance to inhibitory glycine receptor channels. EMBO J. 1992 Dec;11(12):4305–4311. doi: 10.1002/j.1460-2075.1992.tb05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampil I. J., Mason P., Singh H. Anesthetic potency (MAC) is independent of forebrain structures in the rat. Anesthesiology. 1993 Apr;78(4):707–712. doi: 10.1097/00000542-199304000-00014. [DOI] [PubMed] [Google Scholar]
- Sanna E., Harris R. A. Recent developments in alcoholism:neuronal ion channels. Recent Dev Alcohol. 1993;11:169–186. [PubMed] [Google Scholar]
- Sear J. W., Prys-Roberts C. Plasma concentrations of alphaxalone during continuous infusion of Althesin. Br J Anaesth. 1979 Sep;51(9):861–865. doi: 10.1093/bja/51.9.861. [DOI] [PubMed] [Google Scholar]
- Simmonds M. A. Depolarizing responses to glycine, beta-alanine and muscimol in isolated optic nerve and cuneate nucleus. Br J Pharmacol. 1983 Jul;79(3):799–806. doi: 10.1111/j.1476-5381.1983.tb10018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H., Becker C. M., Pritchett D. B., Schofield P. R., Grenningloh G., Kettenmann H., Betz H., Seeburg P. H. Functional chloride channels by mammalian cell expression of rat glycine receptor subunit. Neuron. 1989 May;2(5):1491–1497. doi: 10.1016/0896-6273(89)90195-5. [DOI] [PubMed] [Google Scholar]
- Taleb O., Betz H. Expression of the human glycine receptor alpha 1 subunit in Xenopus oocytes: apparent affinities of agonists increase at high receptor density. EMBO J. 1994 Mar 15;13(6):1318–1324. doi: 10.1002/j.1460-2075.1994.tb06384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg R. J., Handford C. A., Schofield P. R. Distinct agonist- and antagonist-binding sites on the glycine receptor. Neuron. 1992 Sep;9(3):491–496. doi: 10.1016/0896-6273(92)90186-h. [DOI] [PubMed] [Google Scholar]
- Wakamori M., Ikemoto Y., Akaike N. Effects of two volatile anesthetics and a volatile convulsant on the excitatory and inhibitory amino acid responses in dissociated CNS neurons of the rat. J Neurophysiol. 1991 Dec;66(6):2014–2021. doi: 10.1152/jn.1991.66.6.2014. [DOI] [PubMed] [Google Scholar]
- Williams K. L., Ferko A. P., Barbieri E. J., DiGregorio G. J. Glycine enhances the central depressant properties of ethanol in mice. Pharmacol Biochem Behav. 1995 Feb;50(2):199–205. doi: 10.1016/0091-3057(94)00288-t. [DOI] [PubMed] [Google Scholar]


