Abstract
1. The contractile responses to endothelin-1 and the effect on these of various magnesium concentrations, were studied in isolated aortic rings from normotensive Sprague-Dawley rats and deoxycorticosterone acetate-salt (DOCA-salt) hypertensive rats. 2. Contractions induced by endothelin-1 were smaller in endothelium-denuded aortae from DOCA-salt hypertensive rats than in those from normotensive rats. The absence of calcium in the medium attenuated endothelin-1-induced contractions of aortae from both normotensive and DOCA-salt rats, but the contraction was greater in aortae from DOCA-salt hypertensive rats. Ryanodine (which inhibits the release of intracellular calcium) inhibited endothelin-1-induced contractions in aortae from DOCA-salt hypertensive rats to a greater extent than in aortae from normotensive rats. 3. A high extracellular magnesium concentration (4.8 mM) attenuated endothelin-1-induced contractions in tissues from DOCA-salt hypertensive rats but not in tissues from normotensive rats. In the absence of calcium, a high concentration of magnesium attenuated endothelin-1-induced contraction in aortae from both normotensive and hypertensive rats. In the presence of ryanodine, a high concentration of magnesium did not modify the contraction in preparations from either strain. 4. Absence of magnesium attenuated endothelin-1-induced contractions in aortae from both normotensive and DOCA-salt hypertensive rats. In the absence of calcium, removal of magnesium totally inhibited endothelin-1-induced contraction in tissues from normotensive rats but had no effect in those from hypertensive rats. In the presence of ryanodine, the lack of magnesium inhibited endothelin-1-induced contractions in aortae from DOCA-salt hypertensive rats but increased the sensitivity to endothelin-1 of aortae from normotensive rats. 5. The presence of endothelium did not modify the effect of high magnesium on endothelin-1-induced contractions in aortae from normotensive and DOCA-salt hypertensive rats. Conversely, the attenuating effect of magnesium removal on endothelin-1-induced contractions did not occur when endothelium was present. 6. In conclusion, endothelin-1-induced contraction was blunted in aortae from DOCA-salt hypertensive rats. The blunted response was related to altered calcium utilization during contraction. Changes in extracellular magnesium concentration differentially alter endothelin-1-induced contraction in aortae from normotensive and hypertensive rats, possibly by interfering with calcium utilization during contraction. Magnesium may be required for the contractile response to endothelin-1 and increasing magnesium may limit the vascular effects of endothelin-1 in blood vessels from DOCA-salt hypertensive rats.
Full text
PDF

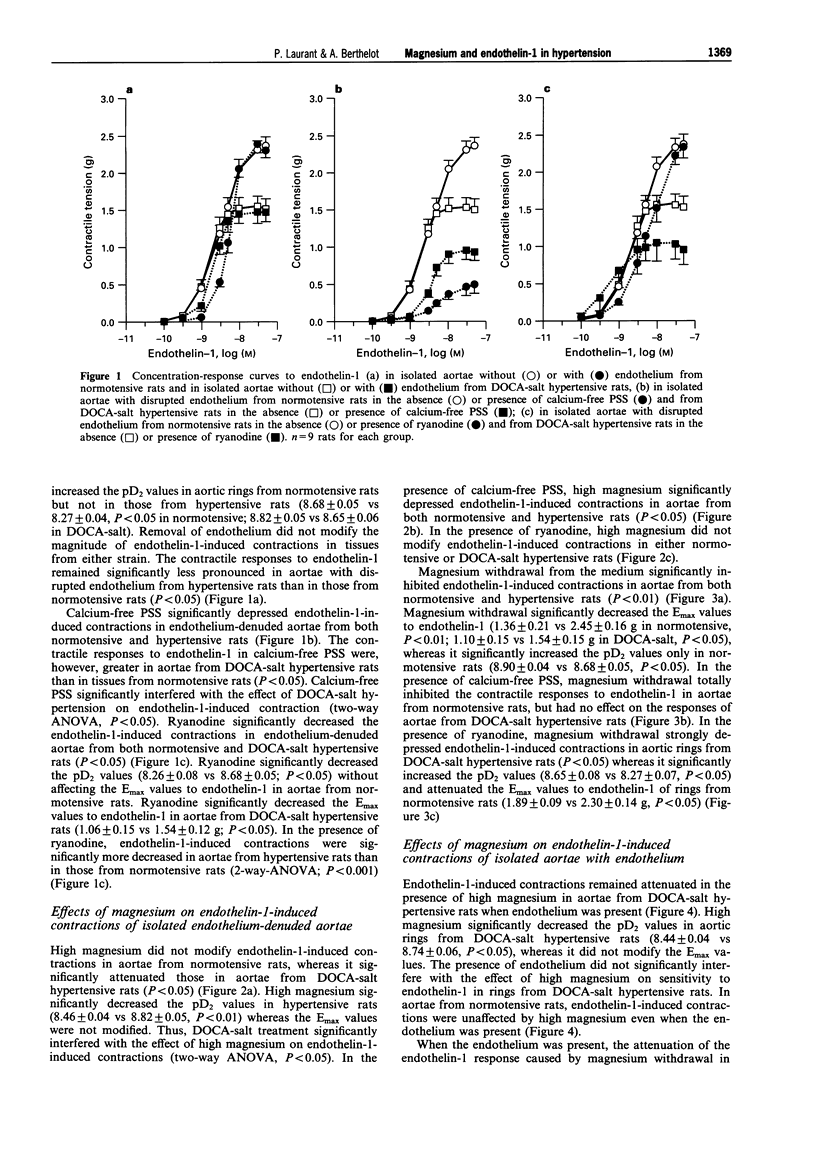
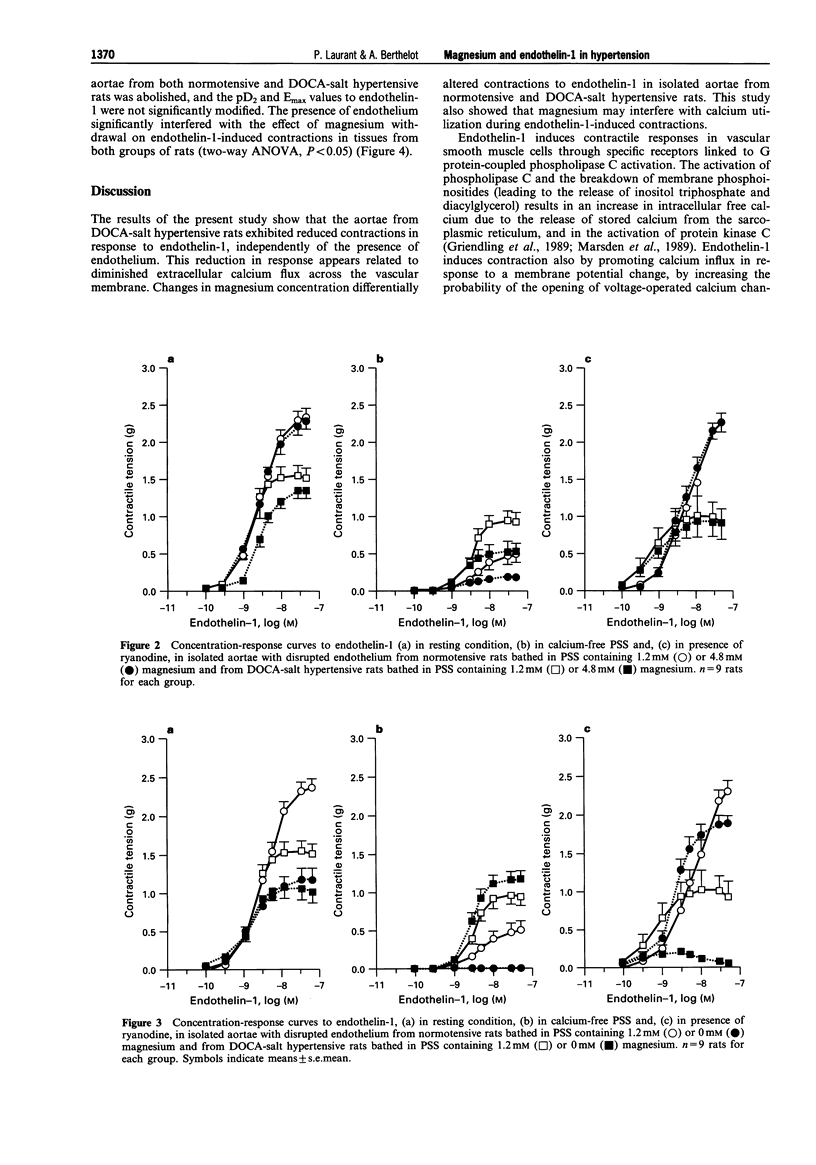
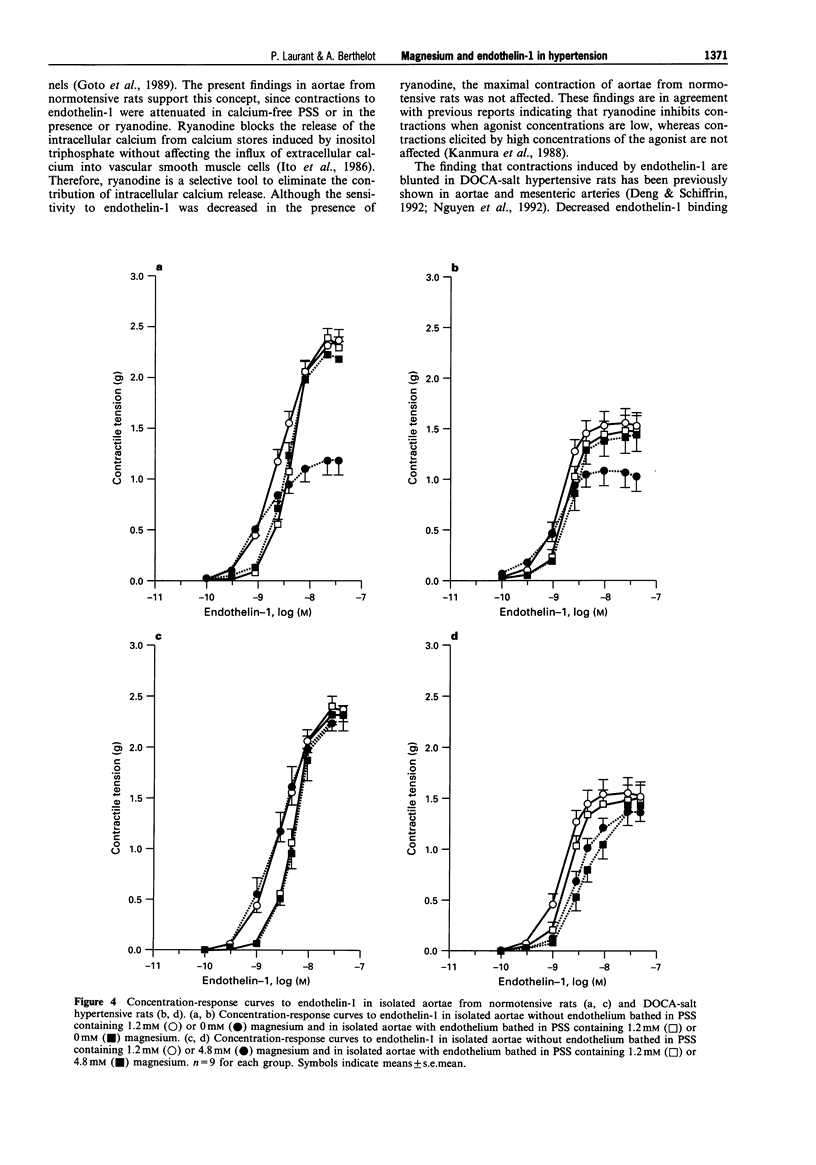



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altura B. M., Altura B. T., Carella A., Gebrewold A., Murakawa T., Nishio A. Mg2+-Ca2+ interaction in contractility of vascular smooth muscle: Mg2+ versus organic calcium channel blockers on myogenic tone and agonist-induced responsiveness of blood vessels. Can J Physiol Pharmacol. 1987 Apr;65(4):729–745. doi: 10.1139/y87-120. [DOI] [PubMed] [Google Scholar]
- Altura B. M., Altura B. T., Gebrewold A., Ising H., Günther T. Magnesium deficiency and hypertension: correlation between magnesium-deficient diets and microcirculatory changes in situ. Science. 1984 Mar 23;223(4642):1315–1317. doi: 10.1126/science.6701524. [DOI] [PubMed] [Google Scholar]
- Altura B. M., Altura B. T. Influence of magnesium on drug-induced contractions and ion content in rabbit aorta. Am J Physiol. 1971 Apr;220(4):938–944. doi: 10.1152/ajplegacy.1971.220.4.938. [DOI] [PubMed] [Google Scholar]
- Altura B. M. Magnesium-neurohypophyseal hormone interactions in contraction of vascular smooth muscle. Am J Physiol. 1975 May;228(5):1615–1620. doi: 10.1152/ajplegacy.1975.228.5.1615. [DOI] [PubMed] [Google Scholar]
- Altura B. T., Altura B. M. Endothelium-dependent relaxation in coronary arteries requires magnesium ions. Br J Pharmacol. 1987 Jul;91(3):449–451. doi: 10.1111/j.1476-5381.1987.tb11235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobik A., Grooms A., Millar J. A., Mitchell A., Grinpukel S. Growth factor activity of endothelin on vascular smooth muscle. Am J Physiol. 1990 Mar;258(3 Pt 1):C408–C415. doi: 10.1152/ajpcell.1990.258.3.C408. [DOI] [PubMed] [Google Scholar]
- Davenport A. P., Ashby M. J., Easton P., Ella S., Bedford J., Dickerson C., Nunez D. J., Capper S. J., Brown M. J. A sensitive radioimmunoassay measuring endothelin-like immunoreactivity in human plasma: comparison of levels in patients with essential hypertension and normotensive control subjects. Clin Sci (Lond) 1990 Mar;78(3):261–264. doi: 10.1042/cs0780261. [DOI] [PubMed] [Google Scholar]
- Deng L. Y., Schiffrin E. L. Effects of endothelin on resistance arteries of DOCA-salt hypertensive rats. Am J Physiol. 1992 Jun;262(6 Pt 2):H1782–H1787. doi: 10.1152/ajpheart.1992.262.6.H1782. [DOI] [PubMed] [Google Scholar]
- Faragó M., Szabó C., Dóra E., Horváth I., Kovách A. G. Contractile and endothelium-dependent dilatory responses of cerebral arteries at various extracellular magnesium concentrations. J Cereb Blood Flow Metab. 1991 Jan;11(1):161–164. doi: 10.1038/jcbfm.1991.20. [DOI] [PubMed] [Google Scholar]
- Flückiger J. P., Nguyen P. V., Li G., Yang X. P., Schiffrin E. L. Calcium, phosphoinositide, and 1,2-diacylglycerol responses of blood vessels of deoxycorticosterone acetate-salt hypertensive rats to endothelin-1. Hypertension. 1992 Jun;19(6 Pt 2):743–748. doi: 10.1161/01.hyp.19.6.743. [DOI] [PubMed] [Google Scholar]
- Fukuda K., Baba A., Kuchii M., Nakamura Y., Nishio I., Masuyama Y. Increased concentration of calcium channel and intracellular free calcium in the acute phase of deoxycorticosterone acetate salt hypertension. J Hypertens Suppl. 1988 Dec;6(4):S261–S263. doi: 10.1097/00004872-198812040-00079. [DOI] [PubMed] [Google Scholar]
- Gold M. E., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G., Ignarro L. J. Antagonistic modulatory roles of magnesium and calcium on release of endothelium-derived relaxing factor and smooth muscle tone. Circ Res. 1990 Feb;66(2):355–366. doi: 10.1161/01.res.66.2.355. [DOI] [PubMed] [Google Scholar]
- Goto K., Kasuya Y., Matsuki N., Takuwa Y., Kurihara H., Ishikawa T., Kimura S., Yanagisawa M., Masaki T. Endothelin activates the dihydropyridine-sensitive, voltage-dependent Ca2+ channel in vascular smooth muscle. Proc Natl Acad Sci U S A. 1989 May;86(10):3915–3918. doi: 10.1073/pnas.86.10.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling K. K., Tsuda T., Alexander R. W. Endothelin stimulates diacylglycerol accumulation and activates protein kinase C in cultured vascular smooth muscle cells. J Biol Chem. 1989 May 15;264(14):8237–8240. [PubMed] [Google Scholar]
- Hirata Y., Takagi Y., Fukuda Y., Marumo F. Endothelin is a potent mitogen for rat vascular smooth muscle cells. Atherosclerosis. 1989 Aug;78(2-3):225–228. doi: 10.1016/0021-9150(89)90227-x. [DOI] [PubMed] [Google Scholar]
- Ito K., Takakura S., Sato K., Sutko J. L. Ryanodine inhibits the release of calcium from intracellular stores in guinea pig aortic smooth muscle. Circ Res. 1986 May;58(5):730–734. doi: 10.1161/01.res.58.5.730. [DOI] [PubMed] [Google Scholar]
- Kanmura Y., Missiaen L., Raeymaekers L., Casteels R. Ryanodine reduces the amount of calcium in intracellular stores of smooth-muscle cells of the rabbit ear artery. Pflugers Arch. 1988 Dec;413(2):153–159. doi: 10.1007/BF00582525. [DOI] [PubMed] [Google Scholar]
- Kemp P. A., Gardiner S. M., Bennett T., Rubin P. C. Magnesium sulphate reverses the carotid vasoconstriction caused by endothelin-I, angiotensin II and neuropeptide-Y, but not that caused by NG-nitro-L-arginine methyl ester, in conscious rats. Clin Sci (Lond) 1993 Aug;85(2):175–181. doi: 10.1042/cs0850175. [DOI] [PubMed] [Google Scholar]
- Kohno M., Yasunari K., Murakawa K., Yokokawa K., Horio T., Fukui T., Takeda T. Plasma immunoreactive endothelin in essential hypertension. Am J Med. 1990 Jun;88(6):614–618. doi: 10.1016/0002-9343(90)90527-k. [DOI] [PubMed] [Google Scholar]
- Ku D. D., Ann H. S. Differential effects of magnesium on basal and agonist-induced EDRF relaxation in canine coronary arteries. J Cardiovasc Pharmacol. 1991 Jun;17(6):999–1006. doi: 10.1097/00005344-199106000-00021. [DOI] [PubMed] [Google Scholar]
- Ku D. D., Ann H. S. Magnesium deficiency produces endothelium-dependent vasorelaxation in canine coronary arteries. J Pharmacol Exp Ther. 1987 Jun;241(3):961–966. [PubMed] [Google Scholar]
- Larivière R., Day R., Schiffrin E. L. Increased expression of endothelin-1 gene in blood vessels of deoxycorticosterone acetate-salt hypertensive rats. Hypertension. 1993 Jun;21(6 Pt 2):916–920. doi: 10.1161/01.hyp.21.6.916. [DOI] [PubMed] [Google Scholar]
- Larivière R., Sventek P., Schiffrin E. L. Expression of endothelin-1 gene in blood vessels of adult spontaneously hypertensive rats. Life Sci. 1995;56(22):1889–1896. doi: 10.1016/0024-3205(95)00163-z. [DOI] [PubMed] [Google Scholar]
- Larivière R., Thibault G., Schiffrin E. L. Increased endothelin-1 content in blood vessels of deoxycorticosterone acetate-salt hypertensive but not in spontaneously hypertensive rats. Hypertension. 1993 Mar;21(3):294–300. doi: 10.1161/01.hyp.21.3.294. [DOI] [PubMed] [Google Scholar]
- Laurant P., Berthelot A. Influence of endothelium on Mg(2+)-induced relaxation in noradrenaline-contracted aorta from DOCA-salt hypertensive rat. Eur J Pharmacol. 1994 Jun 13;258(3):167–172. doi: 10.1016/0014-2999(94)90477-4. [DOI] [PubMed] [Google Scholar]
- Laurant P., Kantelip J. P., Berthelot A. Dietary magnesium supplementation modifies blood pressure and cardiovascular function in mineralocorticoid-salt hypertensive rats but not in normotensive rats. J Nutr. 1995 Apr;125(4):830–841. doi: 10.1093/jn/125.4.830. [DOI] [PubMed] [Google Scholar]
- Li J. S., Larivière R., Schiffrin E. L. Effect of a nonselective endothelin antagonist on vascular remodeling in deoxycorticosterone acetate-salt hypertensive rats. Evidence for a role of endothelin in vascular hypertrophy. Hypertension. 1994 Aug;24(2):183–188. doi: 10.1161/01.hyp.24.2.183. [DOI] [PubMed] [Google Scholar]
- Lüscher T. F., Oemar B. S., Boulanger C. M., Hahn A. W. Molecular and cellular biology of endothelin and its receptors--Part II. J Hypertens. 1993 Feb;11(2):121–126. doi: 10.1097/00004872-199302000-00002. [DOI] [PubMed] [Google Scholar]
- Marsden P. A., Danthuluri N. R., Brenner B. M., Ballermann B. J., Brock T. A. Endothelin action on vascular smooth muscle involves inositol trisphosphate and calcium mobilization. Biochem Biophys Res Commun. 1989 Jan 16;158(1):86–93. doi: 10.1016/s0006-291x(89)80180-9. [DOI] [PubMed] [Google Scholar]
- Meissner G., Henderson J. S. Rapid calcium release from cardiac sarcoplasmic reticulum vesicles is dependent on Ca2+ and is modulated by Mg2+, adenine nucleotide, and calmodulin. J Biol Chem. 1987 Mar 5;262(7):3065–3073. [PubMed] [Google Scholar]
- Mäkynen H., Arvola P., Vapaatalo H., Pörsti I. High calcium diet effectively opposes the development of deoxycorticosterone-salt hypertension in rats. Am J Hypertens. 1994 Jun;7(6):520–528. doi: 10.1093/ajh/7.6.520. [DOI] [PubMed] [Google Scholar]
- Ng L. L., Davies J. E., Ameen M. Intracellular free-magnesium levels in vascular smooth muscle and striated muscle cells of the spontaneously hypertensive rat. Metabolism. 1992 Jul;41(7):772–777. doi: 10.1016/0026-0495(92)90319-6. [DOI] [PubMed] [Google Scholar]
- Nigam S., Averdunk R., Günther T. Alteration of prostanoid metabolism in rats with magnesium deficiency. Prostaglandins Leukot Med. 1986 Jul;23(1):1–10. doi: 10.1016/0262-1746(86)90070-3. [DOI] [PubMed] [Google Scholar]
- Rembold C. M. Regulation of contraction and relaxation in arterial smooth muscle. Hypertension. 1992 Aug;20(2):129–137. doi: 10.1161/01.hyp.20.2.129. [DOI] [PubMed] [Google Scholar]
- Resnick L. M., Gupta R. K., Laragh J. H. Intracellular free magnesium in erythrocytes of essential hypertension: relation to blood pressure and serum divalent cations. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6511–6515. doi: 10.1073/pnas.81.20.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffrin E. L. Endothelin: potential role in hypertension and vascular hypertrophy. Hypertension. 1995 Jun;25(6):1135–1143. doi: 10.1161/01.hyp.25.6.1135. [DOI] [PubMed] [Google Scholar]
- Schiffrin E. L., Thibault G. Plasma endothelin in human essential hypertension. Am J Hypertens. 1991 Apr;4(4 Pt 1):303–308. doi: 10.1093/ajh/4.4.303. [DOI] [PubMed] [Google Scholar]
- Shichiri M., Hirata Y., Ando K., Emori T., Ohta K., Kimoto S., Ogura M., Inoue A., Marumo F. Plasma endothelin levels in hypertension and chronic renal failure. Hypertension. 1990 May;15(5):493–496. doi: 10.1161/01.hyp.15.5.493. [DOI] [PubMed] [Google Scholar]
- Soma M., Cunnane S. C., Horrobin D. F., Manku M. S., Honda M., Hatano M. Effects of low magnesium diet on the vascular prostaglandin and fatty acid metabolism in rats. Prostaglandins. 1988 Oct;36(4):431–441. doi: 10.1016/0090-6980(88)90041-x. [DOI] [PubMed] [Google Scholar]
- Sumner M. J., Cannon T. R., Mundin J. W., White D. G., Watts I. S. Endothelin ETA and ETB receptors mediate vascular smooth muscle contraction. Br J Pharmacol. 1992 Nov;107(3):858–860. doi: 10.1111/j.1476-5381.1992.tb14537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrosa G., Perales A. J., Salom J. B., Miranda F. J., Barberá M. D., Alborch E. Different effects of Mg2+ on endothelin-1- and 5-hydroxytryptamine-elicited responses in goat cerebrovascular bed. J Cardiovasc Pharmacol. 1994 Jun;23(6):1004–1010. doi: 10.1097/00005344-199406000-00020. [DOI] [PubMed] [Google Scholar]
- Tostes R. C., Traub O., Bendhack L. M., Webb R. C. Sarcoplasmic reticulum Ca2+ uptake is not decreased in aorta from deoxycorticosterone acetate hypertensive rats: functional assessment with cyclopiazonic acid. Can J Physiol Pharmacol. 1995 Nov;73(11):1536–1545. doi: 10.1139/y95-212. [DOI] [PubMed] [Google Scholar]
- Touyz R. M., Deng L. Y., Schiffrin E. L. Endothelin subtype B receptor-mediated calcium and contractile responses in small arteries of hypertensive rats. Hypertension. 1995 Dec;26(6 Pt 2):1041–1045. doi: 10.1161/01.hyp.26.6.1041. [DOI] [PubMed] [Google Scholar]
- Touyz R. M., Marshall P. R., Milne F. J. Altered cations and muscle membrane ATPase activity in deoxycorticosterone acetate-salt spontaneously hypertensive rats. J Hypertens. 1991 Aug;9(8):737–750. doi: 10.1097/00004872-199108000-00007. [DOI] [PubMed] [Google Scholar]
- Turlapaty P. D., Altura B. M. Extracellular magnesium ions control calcium exchange and content of vascular smooth muscle. Eur J Pharmacol. 1978 Dec 1;52(3-4):421–423. doi: 10.1016/0014-2999(78)90303-5. [DOI] [PubMed] [Google Scholar]
- Vane J. R., Anggård E. E., Botting R. M. Regulatory functions of the vascular endothelium. N Engl J Med. 1990 Jul 5;323(1):27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- Volpe P., Alderson-Lang B. H., Nickols G. A. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release. I. Effect of Mg2+. Am J Physiol. 1990 Jun;258(6 Pt 1):C1077–C1085. doi: 10.1152/ajpcell.1990.258.6.C1077. [DOI] [PubMed] [Google Scholar]
- Warner T. D., Allcock G. H., Corder R., Vane J. R. Use of the endothelin antagonists BQ-123 and PD 142893 to reveal three endothelin receptors mediating smooth muscle contraction and the release of EDRF. Br J Pharmacol. 1993 Oct;110(2):777–782. doi: 10.1111/j.1476-5381.1993.tb13879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weglicki W. B., Phillips T. M., Freedman A. M., Cassidy M. M., Dickens B. F. Magnesium-deficiency elevates circulating levels of inflammatory cytokines and endothelin. Mol Cell Biochem. 1992 Mar 25;110(2):169–173. doi: 10.1007/BF02454195. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Zhang A., Cheng T. P., Altura B. M. Magnesium regulates intracellular free ionized calcium concentration and cell geometry in vascular smooth muscle cells. Biochim Biophys Acta. 1992 Feb 19;1134(1):25–29. doi: 10.1016/0167-4889(92)90024-6. [DOI] [PubMed] [Google Scholar]
- de Champlain J., Eid H., Papin D. Potentiated endothelin-1-induced phosphoinositide hydrolysis in atria and mesenteric artery of DOCA-salt hypertensive rats. J Hypertens Suppl. 1989 Dec;7(6):S136–S137. doi: 10.1097/00004872-198900076-00064. [DOI] [PubMed] [Google Scholar]
- de Nucci G., Thomas R., D'Orleans-Juste P., Antunes E., Walder C., Warner T. D., Vane J. R. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]


