Abstract
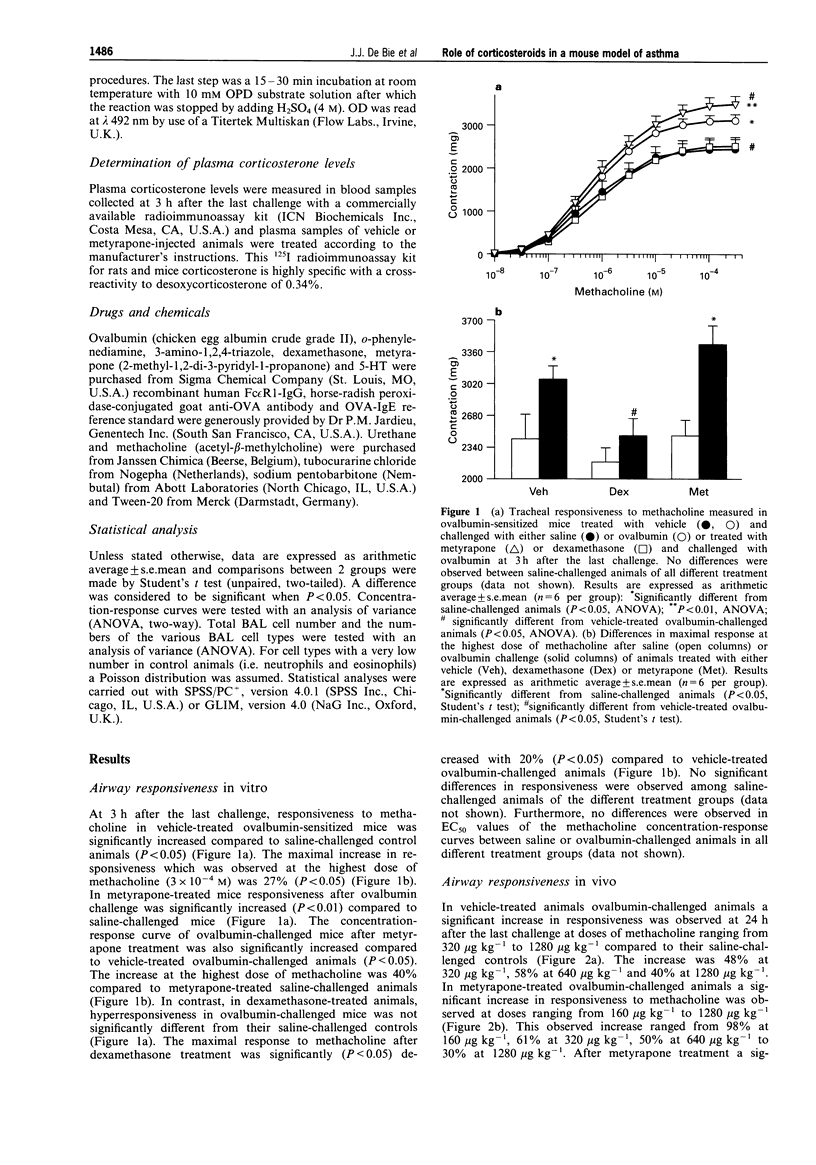
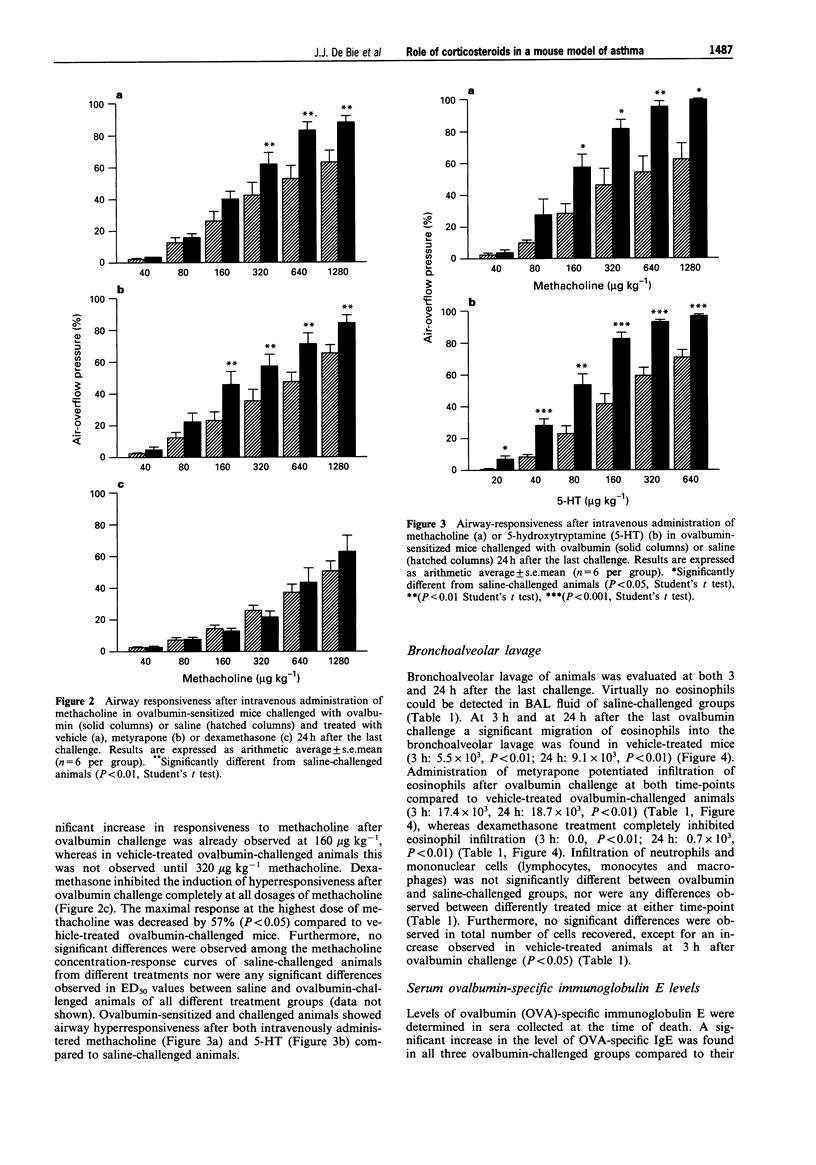
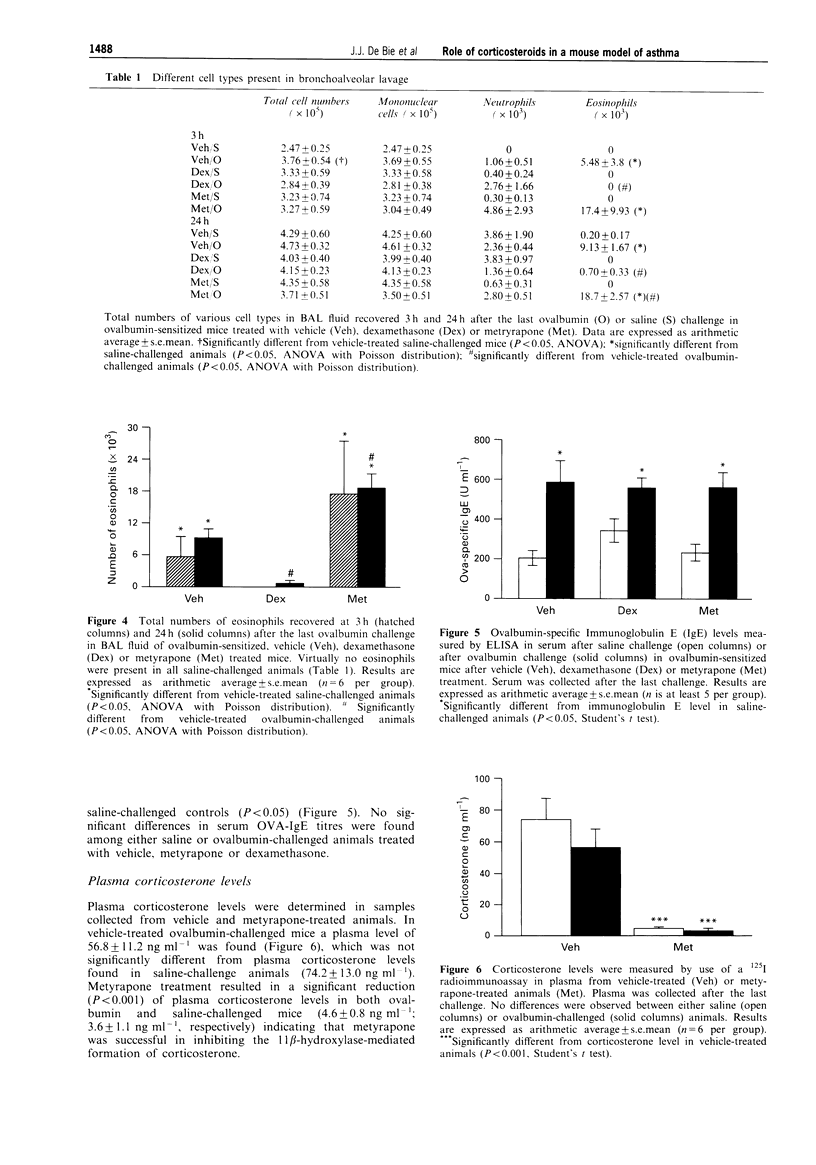
1. Mice were sensitized by 7 intraperitoneal injections of ovalbumin without adjuvant (10 micrograms in 0.5 ml of sterile saline) on alternate days and after 3 weeks exposed to either ovalbumin (2 mg ml-1 in sterile saline) or saline aerosol for 5 min on 8 consecutive days. One day before the first challenge, animals were injected intraperitoneally on a daily basis with vehicle (0.25 ml sterile saline), dexamethasone (0.5 mg kg-1) or metyrapone (30 mg kg-1). 2. In vehicle-treated ovalbumin-sensitized animals ovalbumin challenge induced a significant increase of airway responsiveness to metacholine both in vitro (27%, P < 0.05) and in vivo (40%, P < 0.05) compared to saline-challenged mice. Virtually no eosinophils could be detected after saline challenge, whereas the numbers of eosinophils were significantly increased (P < 0.01) at both 3 and 24 h after the last ovalbumin challenge (5.48 +/- 3.8 x 10(3) and 9.13 +/- 1.7 x 10(3) cells, respectively). Furthermore, a significant increase in ovalbumin-specific immunoglobulin E level (583 +/- 103 units ml-1, P < 0.05) was observed after ovalbumin challenge compared to saline challenge (201 +/- 38 units ml-1). 3. Plasma corticosterone level was significantly reduced (-92%, P < 0.001) after treatment with metyrapone. Treatment with metyrapone significantly increased eosinophil infiltration (17.4 +/- 9.93 x 10(3) and 18.7 +/- 2.57 x 10(3) cells, P < 0.05 at 3 h and 24 h, respectively) and potentiated airway hyperresponsiveness to methacholine compared to vehicle-treated ovalbumin-challenged animals. Dexamethasone inhibited both in vitro and in vivo hyperresponsiveness as well as antigen-induced infiltration of eosinophils (0, P < 0.05 and 0.7 +/- 0.33 x 10(3) cells, P < 0.05 at 3 h and 24 h, respectively). Metyrapone as well as dexamethasone did not affect the increase in ovalbumin-specific immunoglobulin E levels after ovalbumin challenge (565 +/- 70 units/ml-1; P < 0.05; 552 +/- 48 units ml-1, P < 0.05 respectively). 4. From these data it can be concluded that exogenously applied corticosteroids can inhibit eosinophil infiltration as well as airway hyperresponsiveness. Vise versa, endogenously produced corticosteroids play a down-regulating role on the induction of both eosinophil infiltration and airway hyperresponsiveness.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beasley R., Roche W. R., Roberts J. A., Holgate S. T. Cellular events in the bronchi in mild asthma and after bronchial provocation. Am Rev Respir Dis. 1989 Mar;139(3):806–817. doi: 10.1164/ajrccm/139.3.806. [DOI] [PubMed] [Google Scholar]
- Besedovsky H. O., del Rey A., Sorkin E. Lymphokine-containing supernatants from con A-stimulated cells increase corticosterone blood levels. J Immunol. 1981 Jan;126(1):385–387. [PubMed] [Google Scholar]
- Cartier A., Thomson N. C., Frith P. A., Roberts R., Hargreave F. E. Allergen-induced increase in bronchial responsiveness to histamine: relationship to the late asthmatic response and change in airway caliber. J Allergy Clin Immunol. 1982 Sep;70(3):170–177. doi: 10.1016/0091-6749(82)90038-0. [DOI] [PubMed] [Google Scholar]
- Clark T. J., Hetzel M. R. Diurnal variation of asthma. Br J Dis Chest. 1977 Apr;71(2):87–92. doi: 10.1016/0007-0971(77)90087-0. [DOI] [PubMed] [Google Scholar]
- Djukanović R., Roche W. R., Wilson J. W., Beasley C. R., Twentyman O. P., Howarth R. H., Holgate S. T. Mucosal inflammation in asthma. Am Rev Respir Dis. 1990 Aug;142(2):434–457. doi: 10.1164/ajrccm/142.2.434. [DOI] [PubMed] [Google Scholar]
- Elwood W., Barnes P. J., Chung K. F. Airway hyperresponsiveness is associated with inflammatory cell infiltration in allergic brown-Norway rats. Int Arch Allergy Immunol. 1992;99(1):91–97. doi: 10.1159/000236340. [DOI] [PubMed] [Google Scholar]
- Elwood W., Lötvall J. O., Barnes P. J., Chung K. F. Effect of dexamethasone and cyclosporin A on allergen-induced airway hyperresponsiveness and inflammatory cell responses in sensitized Brown-Norway rats. Am Rev Respir Dis. 1992 Jun;145(6):1289–1294. doi: 10.1164/ajrccm/145.6.1289. [DOI] [PubMed] [Google Scholar]
- Fornhem C., Lundberg J. M., Alving K. Allergen-induced late-phase airways obstruction in the pig: the role of endogenous cortisol. Eur Respir J. 1995 Jun;8(6):928–937. [PubMed] [Google Scholar]
- Garssen J., Van Loveren H., Van Der Vliet H., Nijkamp F. P. An isometric method to study respiratory smooth muscle responses in mice. J Pharmacol Methods. 1990 Nov;24(3):209–217. doi: 10.1016/0160-5402(90)90031-f. [DOI] [PubMed] [Google Scholar]
- Hessel E. M., Van Oosterhout A. J., Hofstra C. L., De Bie J. J., Garssen J., Van Loveren H., Verheyen A. K., Savelkoul H. F., Nijkamp F. P. Bronchoconstriction and airway hyperresponsiveness after ovalbumin inhalation in sensitized mice. Eur J Pharmacol. 1995 Dec 7;293(4):401–412. doi: 10.1016/0926-6917(95)90061-6. [DOI] [PubMed] [Google Scholar]
- Iijima H., Ishii M., Yamauchi K., Chao C. L., Kimura K., Shimura S., Shindoh Y., Inoue H., Mue S., Takishima T. Bronchoalveolar lavage and histologic characterization of late asthmatic response in guinea pigs. Am Rev Respir Dis. 1987 Oct;136(4):922–929. doi: 10.1164/ajrccm/136.4.922. [DOI] [PubMed] [Google Scholar]
- Kallenbach J. M., Panz V. R., Joffe B. I., Jankelow D., Anderson R., Haitas B., Seftel H. C. Nocturnal events related to "morning dipping" in bronchial asthma. Chest. 1988 Apr;93(4):751–757. doi: 10.1378/chest.93.4.751. [DOI] [PubMed] [Google Scholar]
- Kung T. T., Jones H., Adams G. K., 3rd, Umland S. P., Kreutner W., Egan R. W., Chapman R. W., Watnick A. S. Characterization of a murine model of allergic pulmonary inflammation. Int Arch Allergy Immunol. 1994 Sep;105(1):83–90. doi: 10.1159/000236807. [DOI] [PubMed] [Google Scholar]
- Mackay T. W., Wallace W. A., Howie S. E., Brown P. H., Greening A. P., Church M. K., Douglas N. J. Role of inflammation in nocturnal asthma. Thorax. 1994 Mar;49(3):257–262. doi: 10.1136/thx.49.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori A., Suko M., Nishizaki Y., Kaminuma O., Matsuzaki G., Ito K., Etoh T., Nakagawa H., Tsuruoka N., Okudaira H. Regulation of interleukin-5 production by peripheral blood mononuclear cells from atopic patients with FK506, cyclosporin A and glucocorticoid. Int Arch Allergy Immunol. 1994;104 (Suppl 1)(1):32–35. doi: 10.1159/000236745. [DOI] [PubMed] [Google Scholar]
- Nijkamp F. P., Flower R. J., Moncada S., Vane J. R. Partial purification of rabbit aorta contracting substance-releasing factor and inhibition of its activity by anti-inflammatory steroids. Nature. 1976 Oct 7;263(5577):479–482. doi: 10.1038/263479a0. [DOI] [PubMed] [Google Scholar]
- O'Byrne P. M., Dolovich J., Hargreave F. E. Late asthmatic responses. Am Rev Respir Dis. 1987 Sep;136(3):740–751. doi: 10.1164/ajrccm/136.3.740. [DOI] [PubMed] [Google Scholar]
- Peers S. H., Duncan G. S., Flower R. J. Development of specific antibody and in vivo response to antigen in different rat strains: effect of dexamethasone and importance of endogenous corticosteroids. Agents Actions. 1993 Jul;39(3-4):174–181. doi: 10.1007/BF01998971. [DOI] [PubMed] [Google Scholar]
- Raeburn D., Underwood S. L., Villamil M. E. Techniques for drug delivery to the airways, and the assessment of lung function in animal models. J Pharmacol Toxicol Methods. 1992 May;27(3):143–159. doi: 10.1016/1056-8719(92)90035-y. [DOI] [PubMed] [Google Scholar]
- Renzi P. M., Olivenstein R., Martin J. G. Effect of dexamethasone on airway inflammation and responsiveness after antigen challenge of the rat. Am Rev Respir Dis. 1993 Oct;148(4 Pt 1):932–939. doi: 10.1164/ajrccm/148.4_Pt_1.932. [DOI] [PubMed] [Google Scholar]
- Richards I. M., Griffin R. L., Shields S. K., Reid M. S., Fidler S. F. Chasing the elusive animal model of late-phase bronchoconstriction: studies in dogs, guinea pigs and rats. Agents Actions. 1992 Nov;37(3-4):178–180. doi: 10.1007/BF02028102. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Fleissner S., Heimann-Weitschat I., Lindstaedt R., Szelenyi I. The effect of different corticosteroids and cyclosporin A on interleukin-4 and interleukin-5 release from murine TH2-type T cells. Eur J Pharmacol. 1994 Aug 1;260(2-3):247–250. doi: 10.1016/0014-2999(94)90345-x. [DOI] [PubMed] [Google Scholar]
- Snyder D. S., Unanue E. R. Corticosteroids inhibit murine macrophage Ia expression and interleukin 1 production. J Immunol. 1982 Nov;129(5):1803–1805. [PubMed] [Google Scholar]
- Van Ommen R., Vredendaal A. E., Savelkoul H. F. Secondary IgE responses in vivo are predominantly generated via gamma 1 epsilon-double positive B cells. Scand J Immunol. 1994 Nov;40(5):491–501. doi: 10.1111/j.1365-3083.1994.tb03495.x. [DOI] [PubMed] [Google Scholar]
- Weitzman E. D., Fukushima D., Nogeire C., Roffwarg H., Gallagher T. F., Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab. 1971 Jul;33(1):14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]


