Abstract
1. The ability of PD 128907 to activate dopamine receptors in the ventral tegmental area, substantia nigra pars compacta, and striatum was investigated by use of in vitro electrophysiological recording and fast cyclic voltammetry. The affinity of a novel D2 selective antagonist L-741,626 for receptors activated by this agonist was measured to determine if its effects were mediated by D2 or D3 receptors. 2. The active (+) enantiomer of PD 128907 bound with high affinity and selectivity to rat D3 dopamine receptors. The Ki values for (+)-PD 128907 were 620 nM at D2, 1 nM at D3 and 720 nM at D4 receptors. 3. (+)-PD 128907 inhibited cell firing in both the ventral tegmental area and substantia nigra pars compacta with EC50 values of 33 nM (pEC50 = 7.48 +/- 0.10, n = 10) and 38 nM (pEC50 = 7.42 +/- 0.15, n = 5), respectively. No effects of (+)-PD 128907 (100 nM) were observed on glutamate or GABA-mediated synaptic potentials elicited by focal bipolar stimulation. 4. L-741,626 antagonized these effects of (+)-PD 128907 in a concentration-dependent and surmountable manner with an affinity, determined from Schild analysis, of 20 nM (pKB = 7.71 +/- 0.14) in the ventral tegmental area and 11 nM (pKB = 7.95 +/- 0.18) in the substantia nigra pars compacta. 5. (+)-PD 128907 also inhibited dopamine release in the caudate-putamen with an EC50 of 66 nM (n = 5). The affinity of L-741,626 for these nerve terminal autoreceptors (pKB = 7.71 +/- 0.06; = 20 nM) was identical to that observed on midbrain dopamine neurones. 6. These data demonstrate that the D3 receptor ligand (+)-PD 128907 is a potent agonist on rat midbrain dopamine neurones. However, its lack of regional selectivity, and the high affinity of the selective D2 receptor antagonist L-741,626 for receptors activated by (+)-PD 128907, was more consistent with an action on D2 autoreceptors rather than upon a D3 dopamine receptor subtype.
Full text
PDF

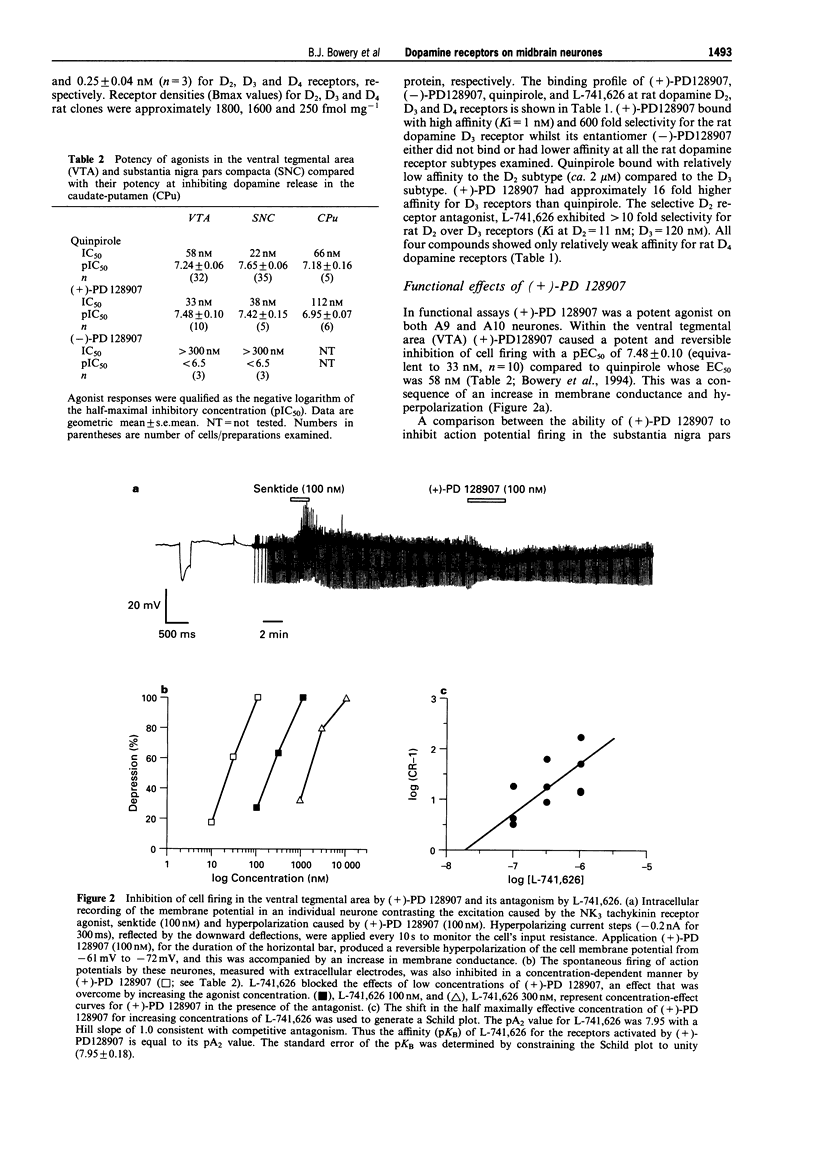
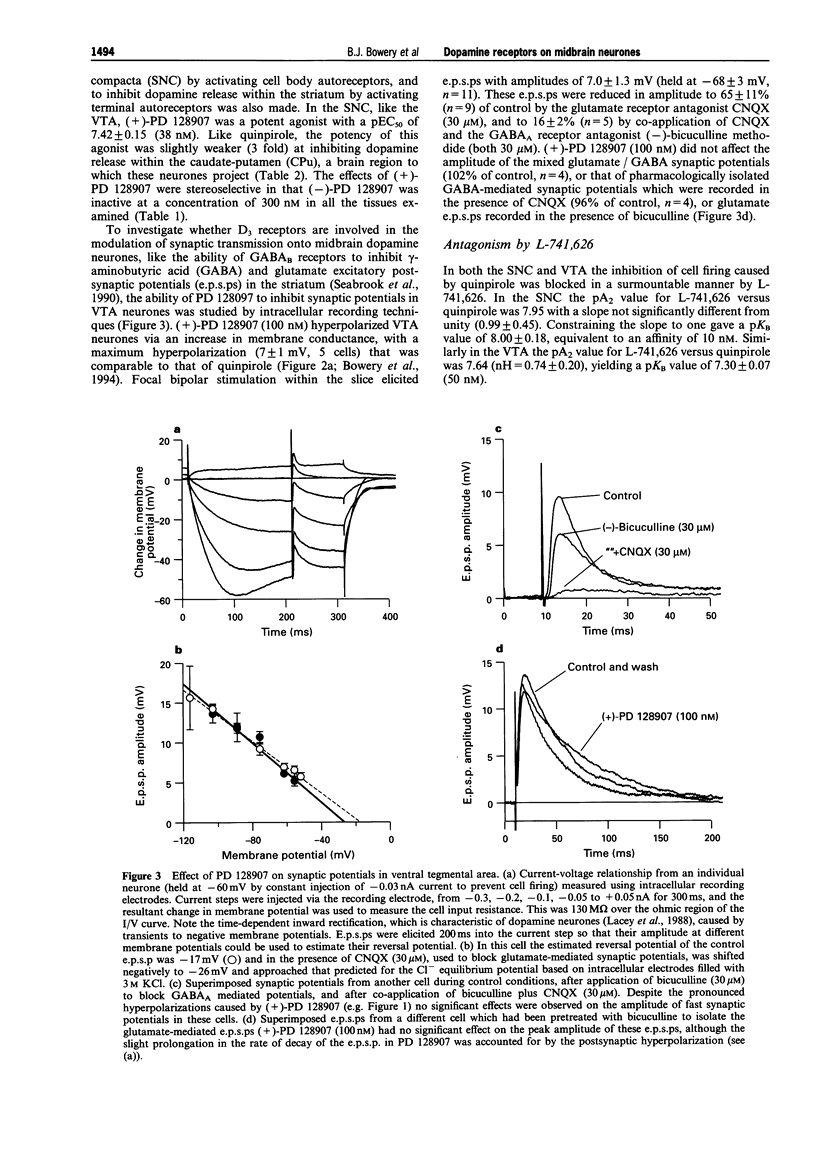
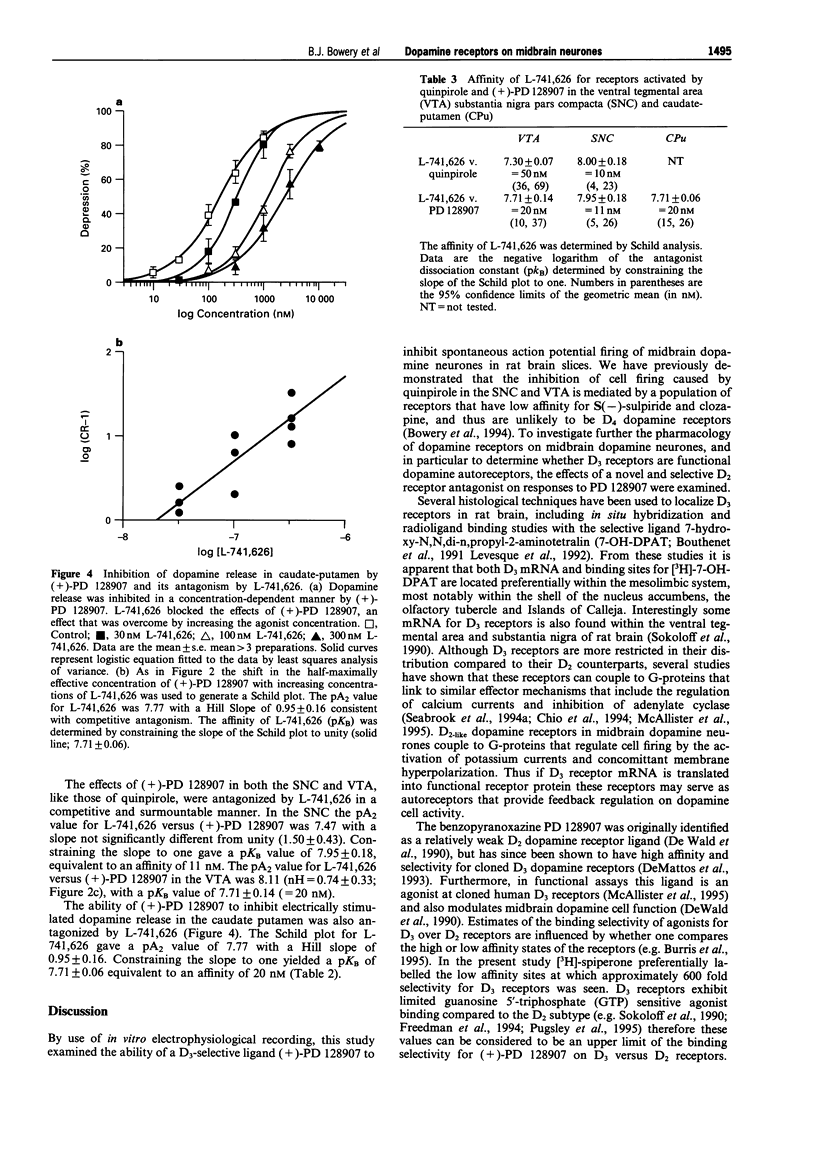


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aretha C. W., Sinha A., Galloway M. P. Dopamine D3-preferring ligands act at synthesis modulating autoreceptors. J Pharmacol Exp Ther. 1995 Aug;274(2):609–613. [PubMed] [Google Scholar]
- Bouthenet M. L., Souil E., Martres M. P., Sokoloff P., Giros B., Schwartz J. C. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991 Nov 15;564(2):203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- Bowery B., Rothwell L. A., Seabrook G. R. Comparison between the pharmacology of dopamine receptors mediating the inhibition of cell firing in rat brain slices through the substantia nigra pars compacta and ventral tegmental area. Br J Pharmacol. 1994 Jul;112(3):873–880. doi: 10.1111/j.1476-5381.1994.tb13161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull D. R., Palij P., Sheehan M. J., Millar J., Stamford J. A., Kruk Z. L., Humphrey P. P. Application of fast cyclic voltammetry to measurement of electrically evoked dopamine overflow from brain slices in vitro. J Neurosci Methods. 1990 Apr;32(1):37–44. doi: 10.1016/0165-0270(90)90069-r. [DOI] [PubMed] [Google Scholar]
- Bunzow J. R., Van Tol H. H., Grandy D. K., Albert P., Salon J., Christie M., Machida C. A., Neve K. A., Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988 Dec 22;336(6201):783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- Burris K. D., Pacheco M. A., Filtz T. M., Kung M. P., Kung H. F., Molinoff P. B. Lack of discrimination by agonists for D2 and D3 dopamine receptors. Neuropsychopharmacology. 1995 Jul;12(4):335–345. doi: 10.1016/0893-133X(94)00099-L. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Chio C. L., Lajiness M. E., Huff R. M. Activation of heterologously expressed D3 dopamine receptors: comparison with D2 dopamine receptors. Mol Pharmacol. 1994 Jan;45(1):51–60. [PubMed] [Google Scholar]
- Cox R. F., Waszczak B. L. Irreversible receptor inactivation reveals differences in dopamine receptor reserve between A9 and A10 dopamine systems: an electrophysiological analysis. Brain Res. 1990 Nov 26;534(1-2):273–282. doi: 10.1016/0006-8993(90)90139-3. [DOI] [PubMed] [Google Scholar]
- DeWald H. A., Heffner T. G., Jaen J. C., Lustgarten D. M., McPhail A. T., Meltzer L. T., Pugsley T. A., Wise L. D. Synthesis and dopamine agonist properties of (+-)-trans-3,4,4a,10b-tetrahydro-4-propyl-2H,5H-[1]benzopyrano [4,3-b]-1,4-oxazin-9-ol and its enantiomers. J Med Chem. 1990 Jan;33(1):445–450. doi: 10.1021/jm00163a068. [DOI] [PubMed] [Google Scholar]
- Freedman S. B., Patel S., Marwood R., Emms F., Seabrook G. R., Knowles M. R., McAllister G. Expression and pharmacological characterization of the human D3 dopamine receptor. J Pharmacol Exp Ther. 1994 Jan;268(1):417–426. [PubMed] [Google Scholar]
- Goldstein M., Deutch A. Y. Dopaminergic mechanisms in the pathogenesis of schizophrenia. FASEB J. 1992 Apr;6(7):2413–2421. [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Drugs and receptors. An overview of the current state of knowledge. Drugs. 1990 Nov;40(5):666–687. doi: 10.2165/00003495-199040050-00003. [DOI] [PubMed] [Google Scholar]
- Kreiss D. S., Bergstrom D. A., Gonzalez A. M., Huang K. X., Sibley D. R., Walters J. R. Dopamine receptor agonist potencies for inhibition of cell firing correlate with dopamine D3 receptor binding affinities. Eur J Pharmacol. 1995 Apr 24;277(2-3):209–214. doi: 10.1016/0014-2999(95)00069-w. [DOI] [PubMed] [Google Scholar]
- Kulagowski J. J., Broughton H. B., Curtis N. R., Mawer I. M., Ridgill M. P., Baker R., Emms F., Freedman S. B., Marwood R., Patel S. 3-((4-(4-Chlorophenyl)piperazin-1-yl)-methyl)-1H-pyrrolo-2,3-b-pyridine: an antagonist with high affinity and selectivity for the human dopamine D4 receptor. J Med Chem. 1996 May 10;39(10):1941–1942. doi: 10.1021/jm9600712. [DOI] [PubMed] [Google Scholar]
- Lacey M. G., Mercuri N. B., North R. A. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol. 1987 Nov;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey M. G., Mercuri N. B., North R. A. On the potassium conductance increase activated by GABAB and dopamine D2 receptors in rat substantia nigra neurones. J Physiol. 1988 Jul;401:437–453. doi: 10.1113/jphysiol.1988.sp017171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque D., Diaz J., Pilon C., Martres M. P., Giros B., Souil E., Schott D., Morgat J. L., Schwartz J. C., Sokoloff P. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8155–8159. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister G., Knowles M. R., Ward-Booth S. M., Sinclair H. A., Patel S., Marwood R., Emms F., Patel S., Smith A., Seabrook G. R. Functional coupling of human D2, D3, and D4 dopamine receptors in HEK293 cells. J Recept Signal Transduct Res. 1995 Jan-Mar;15(1-4):267–281. doi: 10.3109/10799899509045220. [DOI] [PubMed] [Google Scholar]
- Meller E., Helmer-Matyjek E., Bohmaker K., Adler C. H., Friedhoff A. J., Goldstein M. Receptor reserve at striatal dopamine autoreceptors: implications for selectivity of dopamine agonists. Eur J Pharmacol. 1986 Apr 16;123(2):311–314. doi: 10.1016/0014-2999(86)90675-8. [DOI] [PubMed] [Google Scholar]
- Patel J., Trout S. J., Palij P., Whelpton R., Kruk Z. L. Biphasic inhibition of stimulated endogenous dopamine release by 7-OH-DPAT in slices of rat nucleus accumbens. Br J Pharmacol. 1995 Jun;115(3):421–426. doi: 10.1111/j.1476-5381.1995.tb16350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnock R. D. Sensitivity of compacta neurones in the rat substantia nigra slice to dopamine agonists. Eur J Pharmacol. 1983 Dec 23;96(3-4):269–276. doi: 10.1016/0014-2999(83)90316-3. [DOI] [PubMed] [Google Scholar]
- Pugsley T. A., Davis M. D., Akunne H. C., MacKenzie R. G., Shih Y. H., Damsma G., Wikstrom H., Whetzel S. Z., Georgic L. M., Cooke L. W. Neurochemical and functional characterization of the preferentially selective dopamine D3 agonist PD 128907. J Pharmacol Exp Ther. 1995 Dec;275(3):1355–1366. [PubMed] [Google Scholar]
- Reynolds G. P. Developments in the drug treatment of schizophrenia. Trends Pharmacol Sci. 1992 Mar;13(3):116–121. doi: 10.1016/0165-6147(92)90041-4. [DOI] [PubMed] [Google Scholar]
- Seabrook G. R., Howson W., Lacey M. G. Electrophysiological characterization of potent agonists and antagonists at pre- and postsynaptic GABAB receptors on neurones in rat brain slices. Br J Pharmacol. 1990 Dec;101(4):949–957. doi: 10.1111/j.1476-5381.1990.tb14186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook G. R., Kemp J. A., Freedman S. B., Patel S., Sinclair H. A., McAllister G. Functional expression of human D3 dopamine receptors in differentiated neuroblastoma x glioma NG108-15 cells. Br J Pharmacol. 1994 Feb;111(2):391–393. doi: 10.1111/j.1476-5381.1994.tb14746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook G. R., McAllister G., Knowles M. R., Myers J., Sinclair H., Patel S., Freedman S. B., Kemp J. A. Depression of high-threshold calcium currents by activation of human D2 (short) dopamine receptors expressed in differentiated NG108-15 cells. Br J Pharmacol. 1994 Apr;111(4):1061–1066. doi: 10.1111/j.1476-5381.1994.tb14852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook G. R., Patel S., Hutson P. H., Ragan C. I., Bristow L. J. Functional relevance of dopamine receptors. Trends Pharmacol Sci. 1995 Dec;16(12):406–408. doi: 10.1016/s0165-6147(00)89088-4. [DOI] [PubMed] [Google Scholar]
- Sokoloff P., Giros B., Martres M. P., Bouthenet M. L., Schwartz J. C. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990 Sep 13;347(6289):146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Stamford J. A., Kruk Z. L., Millar J. Differential effects of dopamine agonists upon stimulated limbic and striatal dopamine release: in vivo voltammetric data. Br J Pharmacol. 1991 Jan;102(1):45–50. doi: 10.1111/j.1476-5381.1991.tb12130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L., Todd R. D., O'Malley K. L. Dopamine D2 and D3 receptors inhibit dopamine release. J Pharmacol Exp Ther. 1994 Aug;270(2):475–479. [PubMed] [Google Scholar]
- Van Tol H. H., Bunzow J. R., Guan H. C., Sunahara R. K., Seeman P., Niznik H. B., Civelli O. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991 Apr 18;350(6319):610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- Yokoo H., Goldstein M., Meller E. Receptor reserve at striatal dopamine receptors modulating the release of [3H]dopamine. Eur J Pharmacol. 1988 Oct 18;155(3):323–327. doi: 10.1016/0014-2999(88)90523-7. [DOI] [PubMed] [Google Scholar]


