Abstract
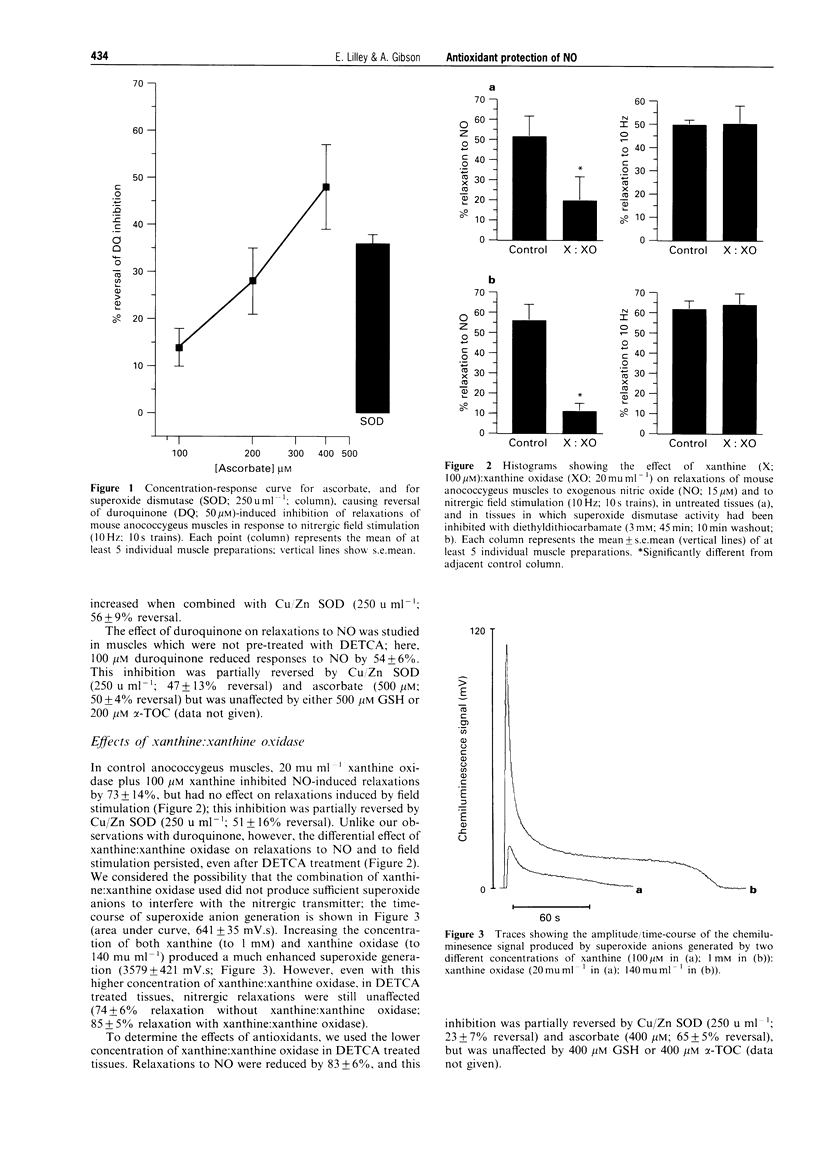
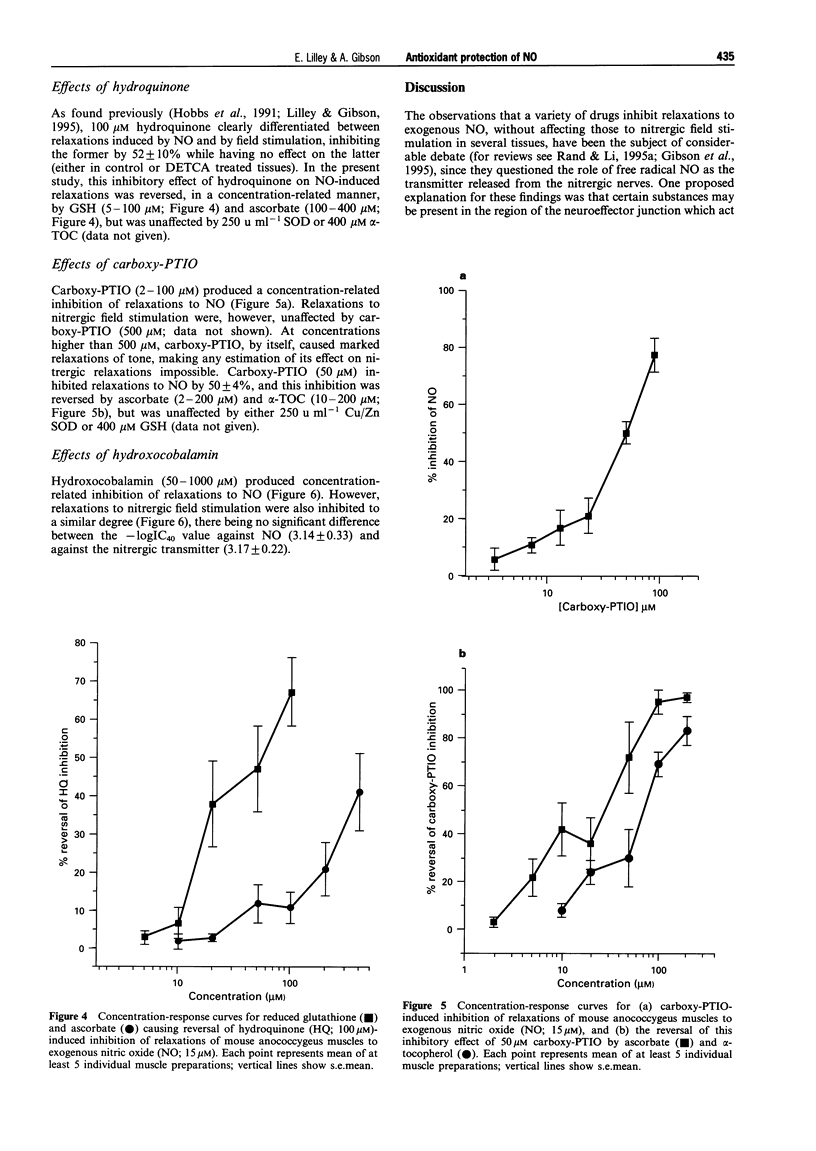
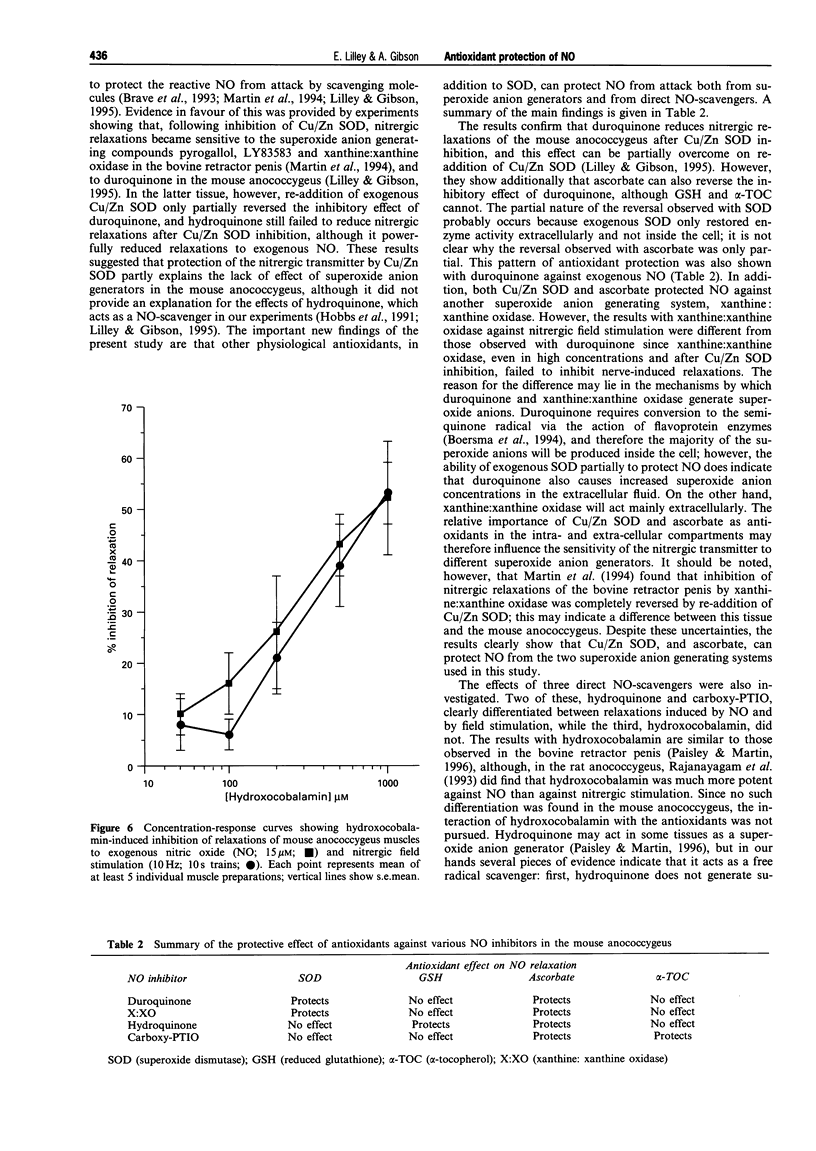
1. The potential protective effect of several antioxidants [Cu/Zn superoxide dismutase (Cu/Zn SOD), ascorbate, reduced glutathione (GSH), and alpha-tocopherol (alpha-TOC)] on relaxations of the mouse anococcygeus muscle to nitric oxide (NO; 15 microM) and, where appropriate, nitrergic field stimulation (10 Hz; 10 s trains) was investigated. 2. The superoxide anion generating drug duroquinone (100 microM) reduced relaxations to exogenous NO by 54 +/- 6%; this inhibition was partially reversed by Cu/Zn SOD (250 u ml-1), and by ascorbate (500 microM). Following inhibition of endogenous Cu/Zn SOD activity with diethyldithiocarbamate (DETCA), duroquinone (50 microM) also reduced relaxations to nitrergic field stimulation (by 53 +/- 6%) and this effect was again reversed by Cu/Zn SOD and by ascorbate. Neither GSH (500 microM) nor alpha-TOC (400 microM) afforded any protection against duroquinone. 3. Xanthine (20 mu ml-1); xanthine oxidase (100 microM) inhibited NO-induced relaxations by 73 +/- 14%, but had no effect on those to nitrergic field stimulation, even after DETCA treatment. The inhibition of exogenous NO was reduced by Cu/Zn SOD (250 u ml-1) and ascorbate (400 microM), but was unaffected by GSH or alpha-TOC (both 400 microM). 4. Hydroquinone (100 microM) also inhibited relaxations to NO (by 52 +/- 10%), but not nitrergic stimulation. In this case, however, the inhibition was reversed by GSH (5-100 microM) and ascorbate (100-400 microM), although Cu/Zn SOD and alpha-TOC were ineffective. 5. 2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (carboxy-PTIO, 50 microM) inhibited NO-induced relaxations by 50 +/- 4%, but had no effect on nitrergic responses; the inhibition was reduced by ascorbate (2-200 microM) and alpha-TOC (10-200 microM), but not by Cu/Zn SOD or GSH. 6. Hydroxocobalamin (5-100 microM) inhibited, equally, relaxations to both NO (-logIC40 3.14 +/- 0.33) and nitrergic stimulation (-logIC40 3.17 +/- 0.22). 7. Thus, a number of physiological antioxidants protected NO from superoxide anions, and from direct NO-scavengers. The possibility that the presence of these antioxidants within nitrergically-innervated tissues might explain the lack of effect of the NO inhibitors on nerve-induced relaxation, without the need to invoke a transmitter other than free radical NO, is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike T., Yoshida M., Miyamoto Y., Sato K., Kohno M., Sasamoto K., Miyazaki K., Ueda S., Maeda H. Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor/.NO through a radical reaction. Biochemistry. 1993 Jan 26;32(3):827–832. doi: 10.1021/bi00054a013. [DOI] [PubMed] [Google Scholar]
- Boersma M. G., Balvers W. G., Boeren S., Vervoort J., Rietjens I. M. NADPH-cytochrome reductase catalysed redox cycling of 1,4-benzoquinone; hampered at physiological conditions, initiated at increased pH values. Biochem Pharmacol. 1994 Jun 1;47(11):1949–1955. doi: 10.1016/0006-2952(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Gibson A., Brave S. R., McFadzean I., Tucker J. F., Wayman C. The nitrergic transmitter of the anococcygeus--NO or not? Arch Int Pharmacodyn Ther. 1995 Jan-Feb;329(1):39–51. [PubMed] [Google Scholar]
- Gibson A., Mirzazadeh S. N-methylhydroxylamine inhibits and M&B 22948 potentiates relaxations of the mouse anococcygeus to non-adrenergic, non-cholinergic field stimulation and to nitrovasodilator drugs. Br J Pharmacol. 1989 Mar;96(3):637–644. doi: 10.1111/j.1476-5381.1989.tb11863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Sheng H. The effects of pyrogallol and hydroquinone on the response to NANC nerve stimulation in the rat anococcygeus and the bovine retractor penis muscles. Br J Pharmacol. 1990 Jan;99(1):194–196. doi: 10.1111/j.1476-5381.1990.tb14677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs A. J., Tucker J. F., Gibson A. Differentiation by hydroquinone of relaxations induced by exogenous and endogenous nitrates in non-vascular smooth muscle: role of superoxide anions. Br J Pharmacol. 1991 Nov;104(3):645–650. doi: 10.1111/j.1476-5381.1991.tb12483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A., Mårtensson J., Mehta T., Krauss A. N., Auld P. A., Meister A. Ascorbic acid prevents oxidative stress in glutathione-deficient mice: effects on lung type 2 cell lamellar bodies, lung surfactant, and skeletal muscle. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5093–5097. doi: 10.1073/pnas.89.11.5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley E., Gibson A. Inhibition of relaxations to nitrergic stimulation of the mouse anococcygeus by duroquinone. Br J Pharmacol. 1995 Dec;116(8):3231–3236. doi: 10.1111/j.1476-5381.1995.tb15129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W., McAllister K. H., Paisley K. NANC neurotransmission in the bovine retractor penis muscle is blocked by superoxide anion following inhibition of superoxide dismutase with diethyldithiocarbamate. Neuropharmacology. 1994 Nov;33(11):1293–1301. doi: 10.1016/0028-3908(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione-ascorbic acid antioxidant system in animals. J Biol Chem. 1994 Apr 1;269(13):9397–9400. [PubMed] [Google Scholar]
- Miele M., Boutelle M. G., Fillenz M. The physiologically induced release of ascorbate in rat brain is dependent on impulse traffic, calcium influx and glutamate uptake. Neuroscience. 1994 Sep;62(1):87–91. doi: 10.1016/0306-4522(94)90316-6. [DOI] [PubMed] [Google Scholar]
- Mårtensson J., Meister A. Glutathione deficiency increases hepatic ascorbic acid synthesis in adult mice. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11566–11568. doi: 10.1073/pnas.89.23.11566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paisley K., Martin W. Blockade of nitrergic transmission by hydroquinone, hydroxocobalamin and carboxy-PTIO in bovine retractor penis: role of superoxide anion. Br J Pharmacol. 1996 Apr;117(8):1633–1638. doi: 10.1111/j.1476-5381.1996.tb15333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajanayagam M. A., Li C. G., Rand M. J. Differential effects of hydroxocobalamin on NO-mediated relaxations in rat aorta and anococcygeus muscle. Br J Pharmacol. 1993 Jan;108(1):3–5. doi: 10.1111/j.1476-5381.1993.tb13429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand M. J., Li C. G. Discrimination by the NO-trapping agent, carboxy-PTIO, between NO and the nitrergic transmitter but not between NO and EDRF. Br J Pharmacol. 1995 Sep;116(2):1906–1910. doi: 10.1111/j.1476-5381.1995.tb16681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand M. J., Li C. G. Nitric oxide as a neurotransmitter in peripheral nerves: nature of transmitter and mechanism of transmission. Annu Rev Physiol. 1995;57:659–682. doi: 10.1146/annurev.ph.57.030195.003303. [DOI] [PubMed] [Google Scholar]
- Rand M. J. Nitrergic transmission: nitric oxide as a mediator of non-adrenergic, non-cholinergic neuro-effector transmission. Clin Exp Pharmacol Physiol. 1992 Mar;19(3):147–169. doi: 10.1111/j.1440-1681.1992.tb00433.x. [DOI] [PubMed] [Google Scholar]


