Abstract
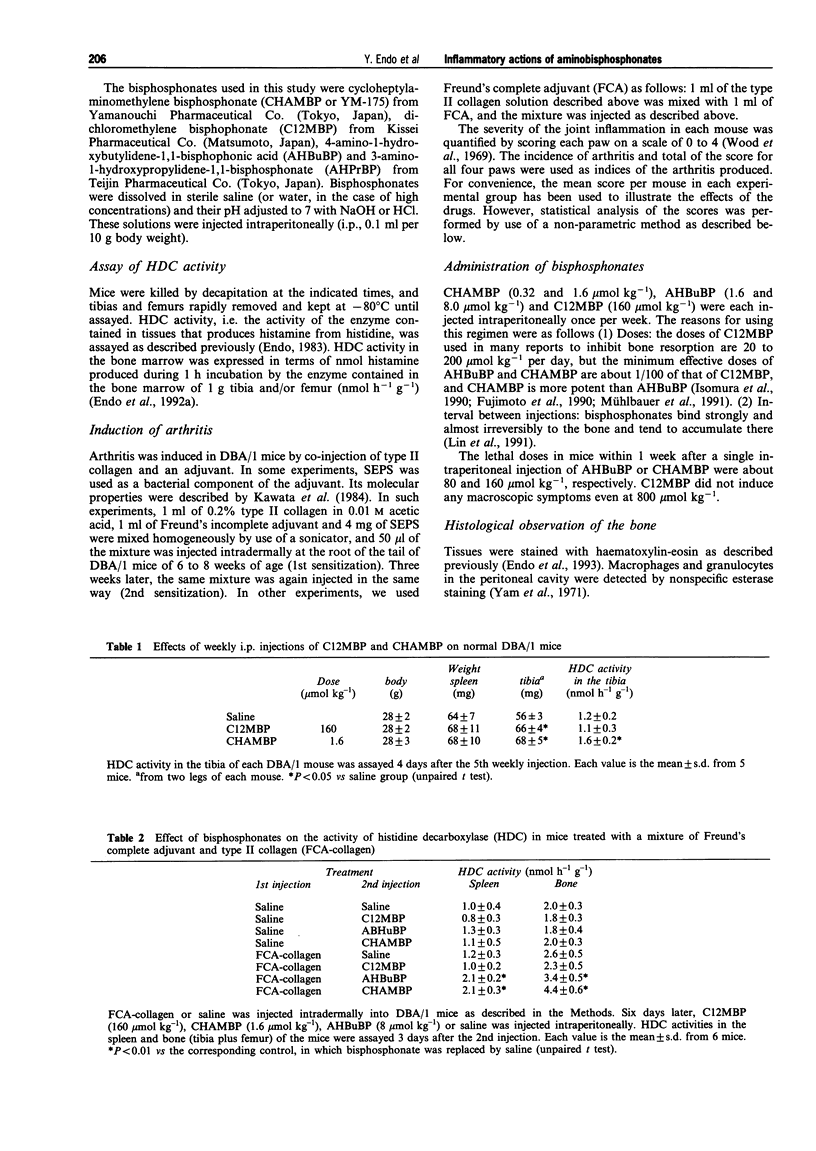
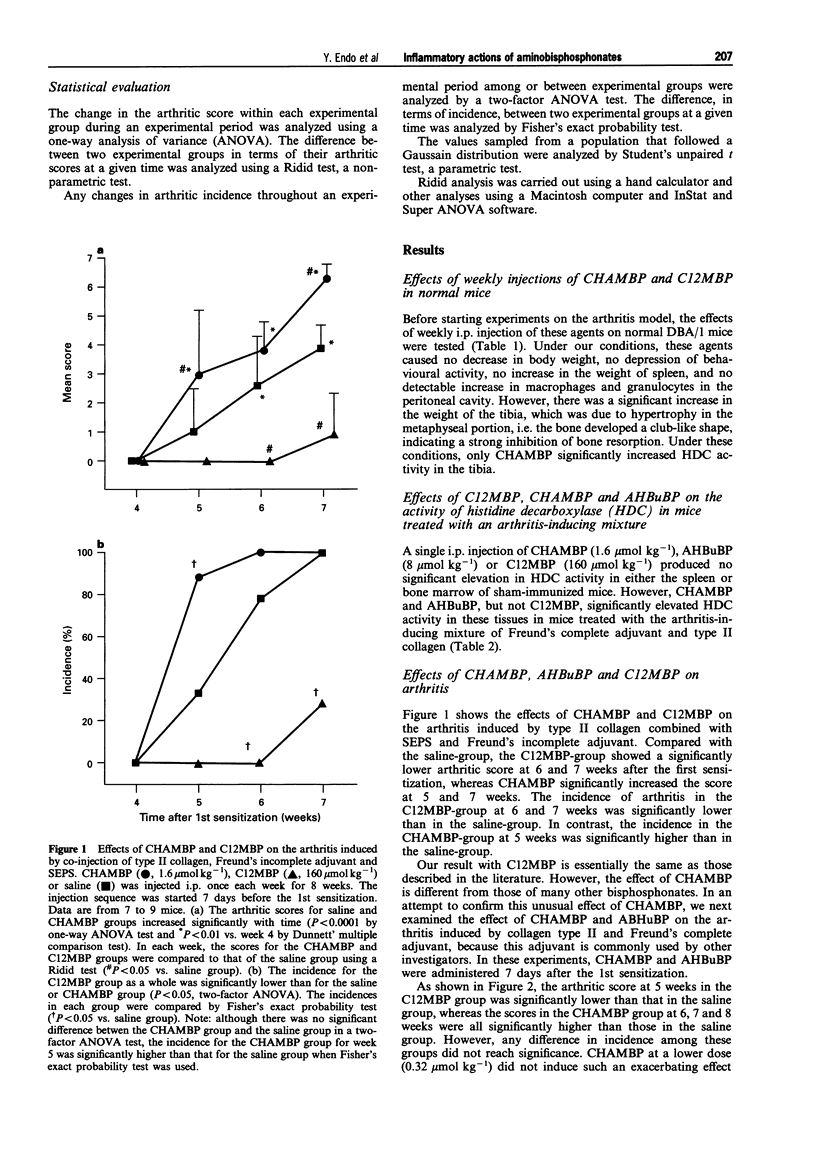
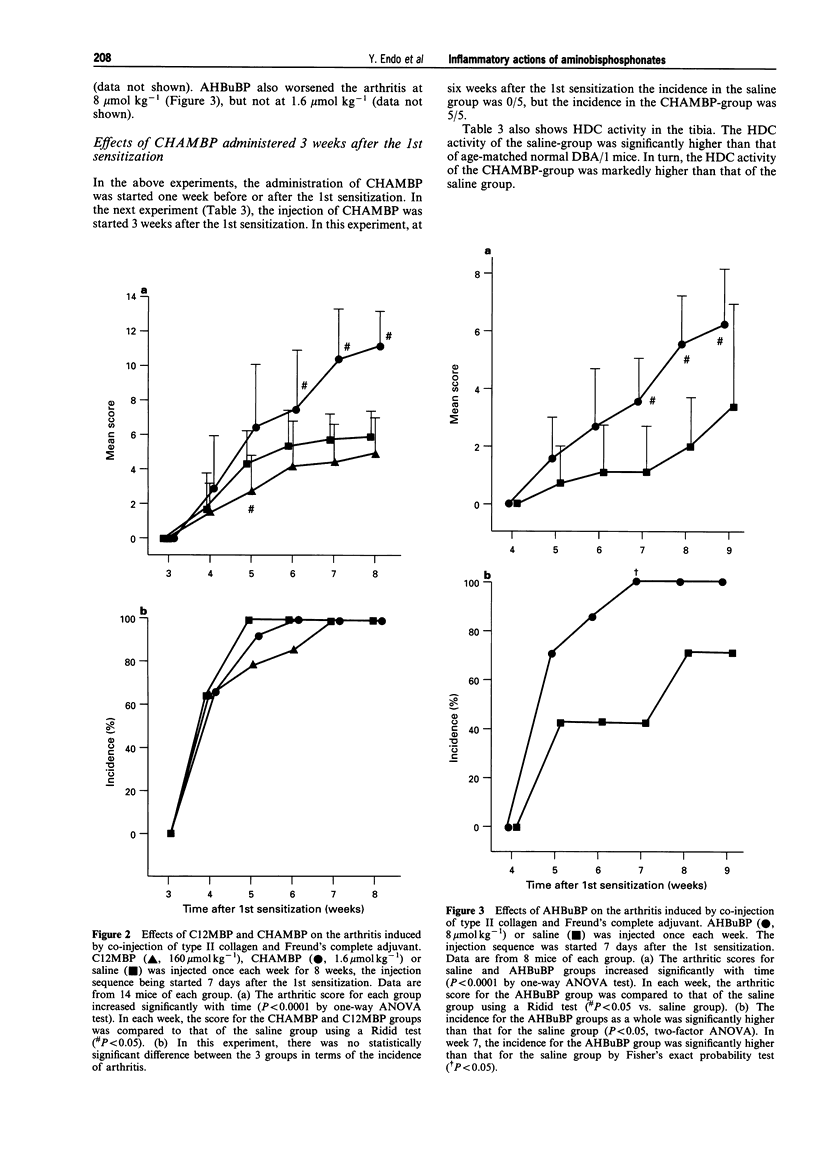
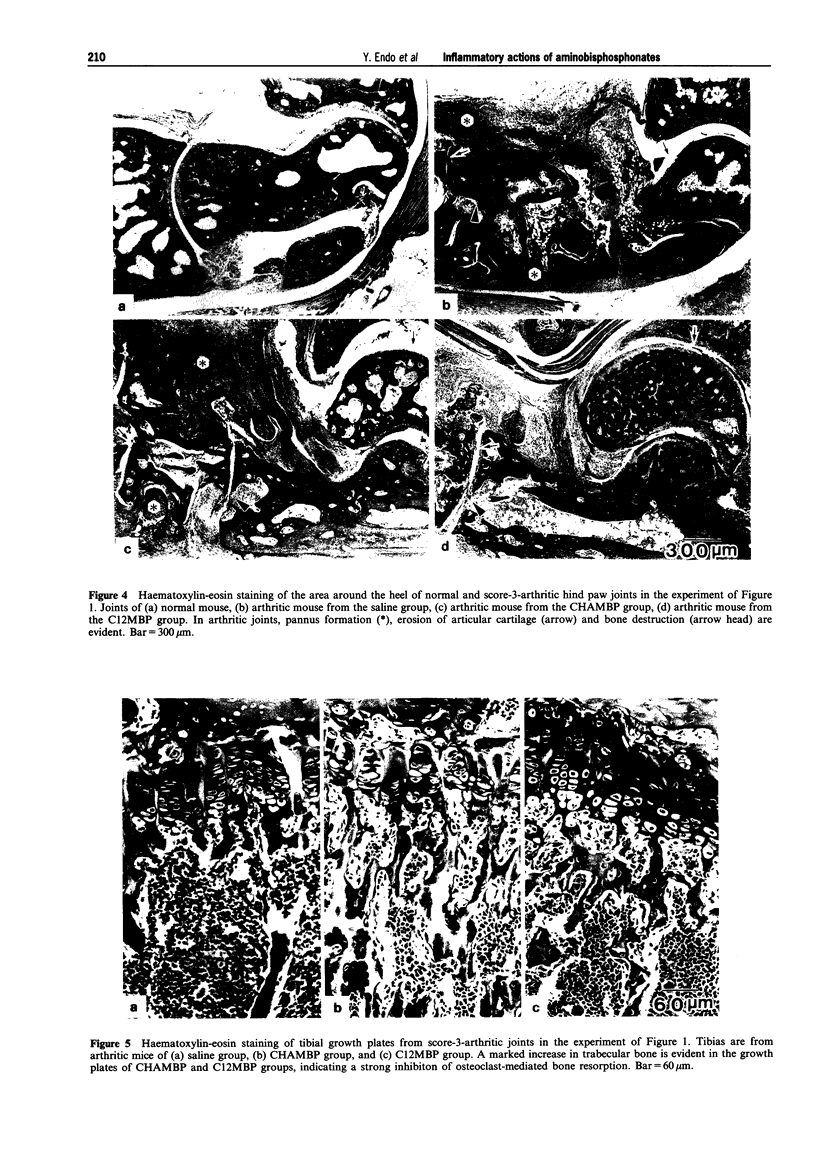
1. Bisphosphonates (BPs) are inhibitors of bone resorption, and many derivatives have been developed for the treatment of enhanced bone resorption. Aminobisphosphonates (aminoBPs) are particularly potent in this respect. We have shown previously that aminoBPs, such as 4-amino-1-hydroxybutylidene-1,1-bisphosphonic acid (AHBuBP), induce histidine decarboxylase, the enzyme forming histamine, and increase macrophages, granulocytes and osteoclast numbers. Non-aminoBPs do not show this activity. 2. In the present study, an additional aminoBP, cycloheptyl-aminomethylene bisphosphonate (CHAMBP), was shown to have similar properties to AHBuBP suggesting that these actions are common among aminoBPs. 3. In experiments carried out to determine if aminoBPs affect immune responses, we found that CHAMBP and AHBuBP each exacerbated the arthritis induced in mice by the co-injection of type II collagen and an adjuvant, a model for rheumatoid arthritis. In contrast, dichloromethylene bisphosphonate (C12MBP), a typical non-aminoBP, did suppress the arthritis. 4. On the basis of these results, and those obtained previously, we propose that the exacerbating effects of CHAMBP and AHBuBP may be related to their ability to stimulate the synthesis of histamine and to increase macrophages and granulocytes. Conversely, we propose that the suppressive effect of C12MBP on arthritis is related to its cytotoxic action on macrophages or granulocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adami S., Bhalla A. K., Dorizzi R., Montesanti F., Rosini S., Salvagno G., Lo Cascio V. The acute-phase response after bisphosphonate administration. Calcif Tissue Int. 1987 Dec;41(6):326–331. doi: 10.1007/BF02556671. [DOI] [PubMed] [Google Scholar]
- Ando T., Endo Y., Abe M., Kumagai K. Stimulation of the synthesis of histamine and putrescine in mice by a peptidoglycan of gram-positive bacteria. Microbiol Immunol. 1994;38(3):209–215. doi: 10.1111/j.1348-0421.1994.tb01766.x. [DOI] [PubMed] [Google Scholar]
- Barbier A., Breliére J. C., Remandet B., Roncucci R. Studies on the chronic phase of adjuvant arthritis: effect of SR 41319, a new diphosphonate. Ann Rheum Dis. 1986 Jan;45(1):67–74. doi: 10.1136/ard.45.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijvoet O. L., Frijlink W. B., Jie K., van der Linden H., Meijer C. J., Mulder H., van Paassen H. C., Reitsma P. H., te Velde J., de Vries E. APD in Paget's disease of bone. Role of the mononuclear phagocyte system? Arthritis Rheum. 1980 Oct;23(10):1193–1204. doi: 10.1002/art.1780231018. [DOI] [PubMed] [Google Scholar]
- Byron J. W. Mechanism for histamine H2-receptor induced cell-cycle changes in the bone marrow stem cell. Agents Actions. 1977 Jul;7(2):209–213. doi: 10.1007/BF01969974. [DOI] [PubMed] [Google Scholar]
- Chambers T. J. Diphosphonates inhibit bone resorption by macrophages in vitro. J Pathol. 1980 Nov;132(3):255–262. doi: 10.1002/path.1711320307. [DOI] [PubMed] [Google Scholar]
- Dunn C. J., Galinet L. A., Wu H., Nugent R. A., Schlachter S. T., Staite N. D., Aspar D. G., Elliott G. A., Essani N. A., Rohloff N. A. Demonstration of novel anti-arthritic and anti-inflammatory effects of diphosphonates. J Pharmacol Exp Ther. 1993 Sep;266(3):1691–1698. [PubMed] [Google Scholar]
- Dy M., Machavoine F., Lebel B., Ichikawa A., Gastinel L. N., Schneider E. Interleukin 3 promotes histamine synthesis in hematopoietic progenitors by increasing histidine decarboxylase mRNA expression. Biochem Biophys Res Commun. 1993 Apr 15;192(1):167–173. doi: 10.1006/bbrc.1993.1396. [DOI] [PubMed] [Google Scholar]
- Endo Y. A simple method for the determination of polyamines and histamine and its application to the assay of ornithine and histidine decarboxylase activities. Methods Enzymol. 1983;94:42–47. doi: 10.1016/s0076-6879(83)94008-9. [DOI] [PubMed] [Google Scholar]
- Endo Y. Induction of histidine and ornithine decarboxylase activities in mouse tissues by recombinant interleukin-1 and tumor necrosis factor. Biochem Pharmacol. 1989 Apr 15;38(8):1287–1292. doi: 10.1016/0006-2952(89)90335-3. [DOI] [PubMed] [Google Scholar]
- Endo Y., Kikuchi T., Nakamura M., Shinoda H. Determination of histamine and polyamines in calcified tissues of mice: contribution of mast cells and histidine decarboxylase to the amount of histamine in the bone. Calcif Tissue Int. 1992 Jul;51(1):67–71. doi: 10.1007/BF00296220. [DOI] [PubMed] [Google Scholar]
- Endo Y., Kikuchi T., Takeda Y., Nitta Y., Rikiishi H., Kumagai K. GM-CSF and G-CSF stimulate the synthesis of histamine and putrescine in the hematopoietic organs in vivo. Immunol Lett. 1992 Jun;33(1):9–13. doi: 10.1016/0165-2478(92)90086-4. [DOI] [PubMed] [Google Scholar]
- Endo Y., Nakamura M., Kikuchi T., Shinoda H., Takeda Y., Nitta Y., Kumagai K. Aminoalkylbisphosphonates, potent inhibitors of bone resorption, induce a prolonged stimulation of histamine synthesis and increase macrophages, granulocytes, and osteoclasts in vivo. Calcif Tissue Int. 1993 Mar;52(3):248–254. doi: 10.1007/BF00298728. [DOI] [PubMed] [Google Scholar]
- Endo Y., Nakamura M., Nitta Y., Kumagai K. Effects of macrophage depletion on the induction of histidine decarboxylase by lipopolysaccharide, interleukin 1 and tumour necrosis factor. Br J Pharmacol. 1995 Jan;114(1):187–193. doi: 10.1111/j.1476-5381.1995.tb14924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan A. M., Chambers T. J. Dichloromethylenebisphosphonate (Cl2MBP) inhibits bone resorption through injury to osteoclasts that resorb Cl2MBP-coated bone. Bone Miner. 1989 Apr;6(1):33–43. doi: 10.1016/0169-6009(89)90021-4. [DOI] [PubMed] [Google Scholar]
- Flora L. Comparative antiinflammatory and bone protective effects of two diphosphonates in adjuvant arthritis. Arthritis Rheum. 1979 Apr;22(4):340–346. doi: 10.1002/art.1780220405. [DOI] [PubMed] [Google Scholar]
- Francis M. D., Flora L., King W. R. The effects of disodium ethane-1-hydroxy-1, 1-diphosphonate on adjuvant induced arthritis in rats. Calcif Tissue Res. 1972;9(2):109–121. doi: 10.1007/BF02061949. [DOI] [PubMed] [Google Scholar]
- Francis M. D., Hovancik K., Boyce R. W. NE-58095: a diphosphonate which prevents bone erosion and preserves joint architecture in experimental arthritis. Int J Tissue React. 1989;11(5):239–252. [PubMed] [Google Scholar]
- Green J. R., Müller K., Jaeggi K. A. Preclinical pharmacology of CGP 42'446, a new, potent, heterocyclic bisphosphonate compound. J Bone Miner Res. 1994 May;9(5):745–751. doi: 10.1002/jbmr.5650090521. [DOI] [PubMed] [Google Scholar]
- Lebel B., Schneider E., Piquet-Pellorce C., Machavoine F., Kindler V., Luffau G., Dy M. Antigenic challenge of immunized mice induces endogeneous production of IL-3 that increases histamine synthesis in hematopoietic organs. J Immunol. 1990 Aug 15;145(4):1222–1226. [PubMed] [Google Scholar]
- Liberman U. A., Weiss S. R., Bröll J., Minne H. W., Quan H., Bell N. H., Rodriguez-Portales J., Downs R. W., Jr, Dequeker J., Favus M. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med. 1995 Nov 30;333(22):1437–1443. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- Lin J. H., Duggan D. E., Chen I. W., Ellsworth R. L. Physiological disposition of alendronate, a potent anti-osteolytic bisphosphonate, in laboratory animals. Drug Metab Dispos. 1991 Sep-Oct;19(5):926–932. [PubMed] [Google Scholar]
- Maccagno A., Di Giorgio E., Roldan E. J., Caballero L. E., Perez Lloret A. Double blind radiological assessment of continuous oral pamidronic acid in patients with rheumatoid arthritis. Scand J Rheumatol. 1994;23(4):211–214. doi: 10.3109/03009749409103063. [DOI] [PubMed] [Google Scholar]
- Markusse H. M., Lafeber G. J., Breedveld F. C. Bisphosphonates in collagen arthritis. Rheumatol Int. 1990;9(6):281–283. doi: 10.1007/BF00541325. [DOI] [PubMed] [Google Scholar]
- Mühlbauer R. C., Bauss F., Schenk R., Janner M., Bosies E., Strein K., Fleisch H. BM 21.0955, a potent new bisphosphonate to inhibit bone resorption. J Bone Miner Res. 1991 Sep;6(9):1003–1011. doi: 10.1002/jbmr.5650060915. [DOI] [PubMed] [Google Scholar]
- Nakaya N., Tasaka K. The influence of histamine on precursors of granulocytic leukocytes in murine bone marrow. Life Sci. 1988;42(9):999–1010. doi: 10.1016/0024-3205(88)90430-4. [DOI] [PubMed] [Google Scholar]
- Nugent R. A., Murphy M., Schlachter S. T., Dunn C. J., Smith R. J., Staite N. D., Galinet L. A., Shields S. K., Aspar D. G., Richard K. A. Pyrazoline bisphosphonate esters as novel antiinflammatory and antiarthritic agents. J Med Chem. 1993 Jan 8;36(1):134–139. doi: 10.1021/jm00053a017. [DOI] [PubMed] [Google Scholar]
- Ralston S. H., Hacking L., Willocks L., Bruce F., Pitkeathly D. A. Clinical, biochemical, and radiographic effects of aminohydroxypropylidene bisphosphonate treatment in rheumatoid arthritis. Ann Rheum Dis. 1989 May;48(5):396–399. doi: 10.1136/ard.48.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitsma P. H., Teitelbaum S. L., Bijvoet O. L., Kahn A. J. Differential action of the bisphosphonates (3-amino-1-hydroxypropylidene)-1,1-bisphosphonate (APD) and disodium dichloromethylidene bisphosphonate (Cl2MDP) on rat macrophage-mediated bone resorption in vitro. J Clin Invest. 1982 Nov;70(5):927–933. doi: 10.1172/JCI110704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E., Piquet-Pellorce C., Dy M. New role for histamine in interleukin-3-induced proliferation of hematopoietic stem cells. J Cell Physiol. 1990 May;143(2):337–343. doi: 10.1002/jcp.1041430218. [DOI] [PubMed] [Google Scholar]
- Tan P. L., Ames R., Yeoman S., Ibbertson H. K., Caughey D. E. Effects of aminobisphosphonate infusion on biochemical indices of bone metabolism in rheumatoid arthritis. Br J Rheumatol. 1989 Aug;28(4):325–328. doi: 10.1093/rheumatology/28.4.325. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N., Kors N., vd Ende M., Dijkstra C. D. Depletion and repopulation of macrophages in spleen and liver of rat after intravenous treatment with liposome-encapsulated dichloromethylene diphosphonate. Cell Tissue Res. 1990 May;260(2):215–222. doi: 10.1007/BF00318625. [DOI] [PubMed] [Google Scholar]
- Wood F. D., Pearson C. M., Tanaka A. Capacity of mycobacterial wax D and its subfractions to induce adjuvant arthritis in rats. Int Arch Allergy Appl Immunol. 1969;35(5):456–467. doi: 10.1159/000230198. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Yi S. N., Xu Y. H. The influence of histamine at various concentrations on the cell cycle state of hematopoietic stem cells (CFU-s). Int J Cell Cloning. 1988 Jul;6(4):290–295. doi: 10.1002/stem.5530060406. [DOI] [PubMed] [Google Scholar]
- van Rooijen N., Kors N. Effects of intracellular diphosphonates on cells of the mononuclear phagocyte system: in vivo effects of liposome-encapsulated diphosphonates on different macrophage subpopulations in the spleen. Calcif Tissue Int. 1989 Sep;45(3):153–156. doi: 10.1007/BF02556058. [DOI] [PubMed] [Google Scholar]
- van Rooijen N., van Nieuwmegen R. Elimination of phagocytic cells in the spleen after intravenous injection of liposome-encapsulated dichloromethylene diphosphonate. An enzyme-histochemical study. Cell Tissue Res. 1984;238(2):355–358. doi: 10.1007/BF00217308. [DOI] [PubMed] [Google Scholar]




