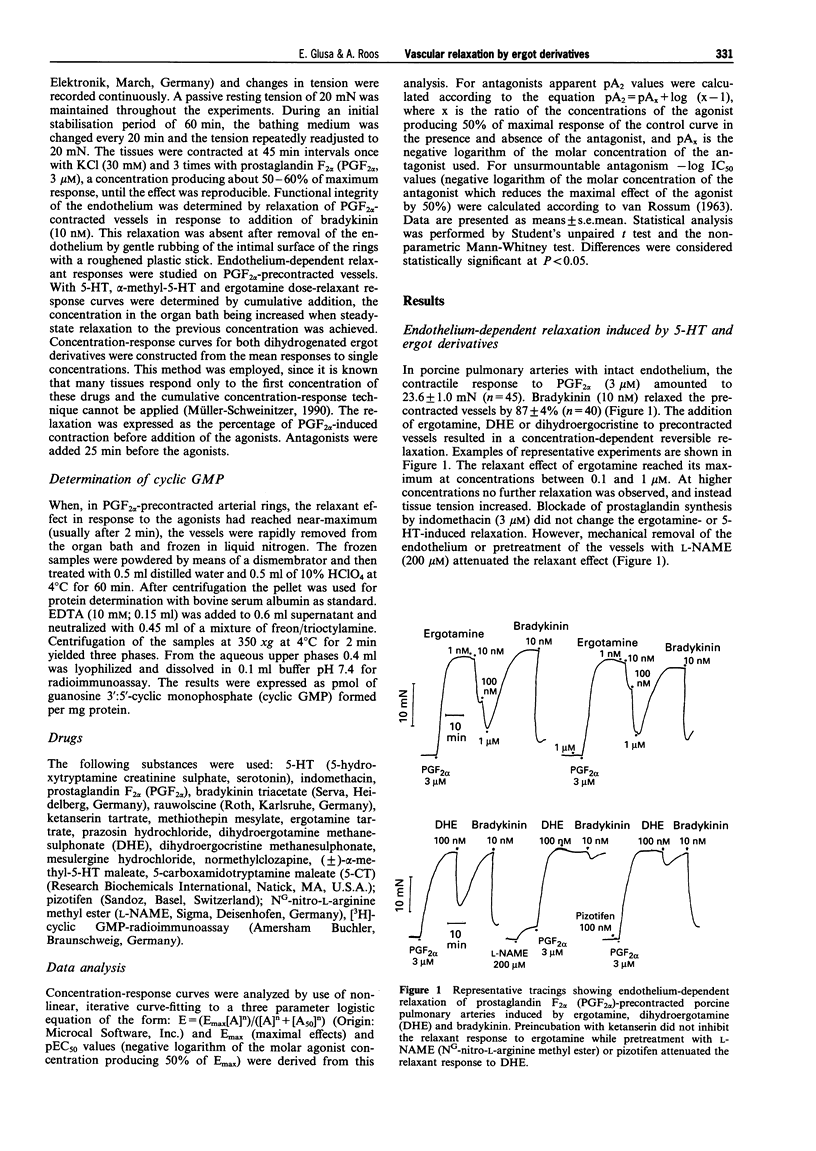
Abstract
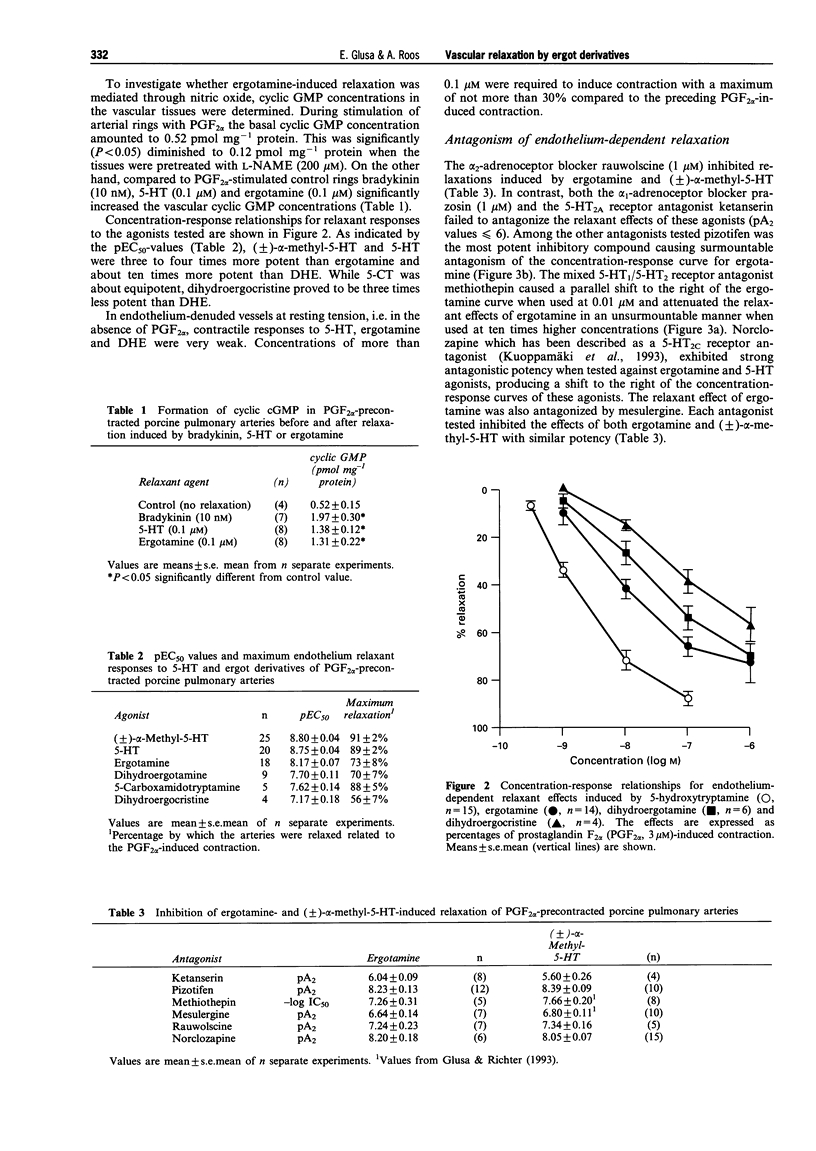
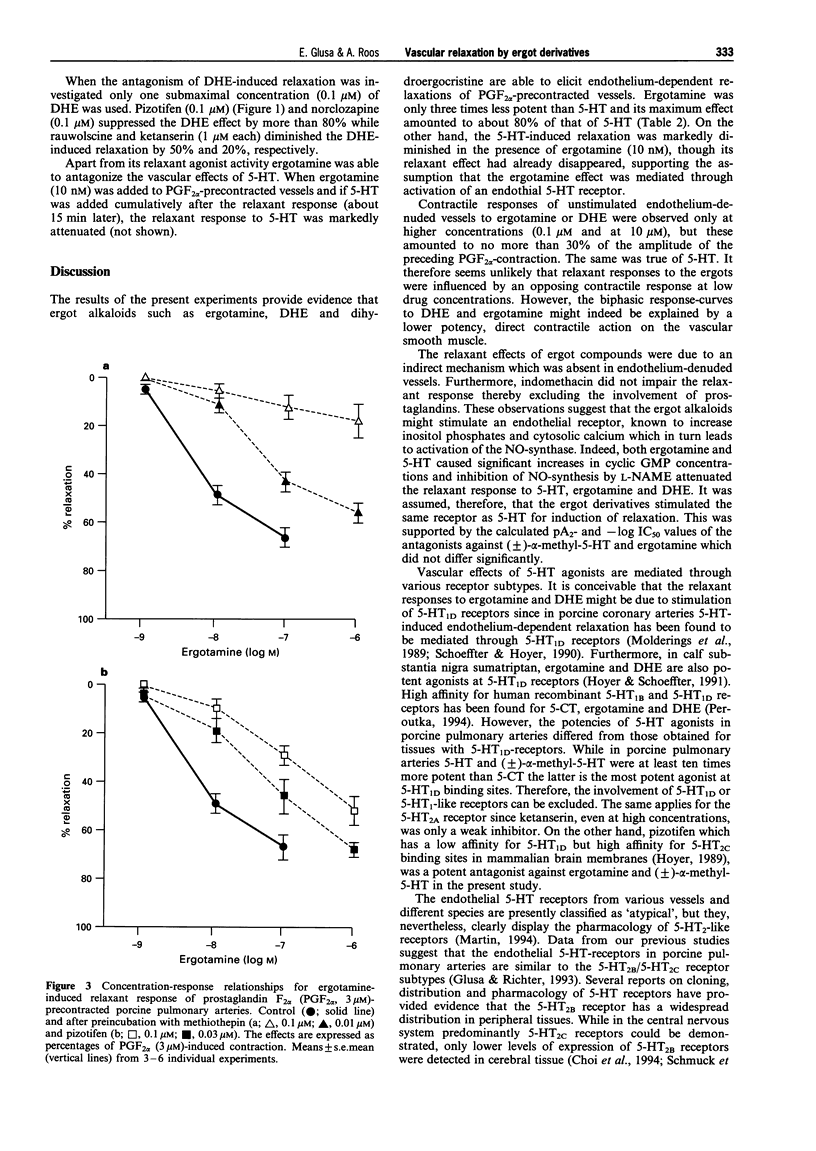
1. The aim of the present study was to investigate whether antimigraine ergot compounds may act at endothelial 5-hydroxytryptamine (5-HT) receptors which trigger the release of endothelium-derived relaxing factor (EDRF). Changes in tone of porcine isolated pulmonary arteries were measured isometrically. The integrity of the endothelium was assessed by the bradykinin-induced relaxation of prostaglandin F2 alpha (PGF2 alpha, 3 microM)-precontracted vessels. 2. The ergot derivatives ergotamine, dihydroergotamine (DHE) and dihydroergocristine, as well as 5-HT and (+/-)-alpha-methyl-5-HT, elicited a reversible endothelium-dependent relaxation of PGF2 alpha-precontracted arterial ring segments. The relaxation to both ergotamine and 5-HT was associated with an increase in cyclic GMP. After pretreatment of the vessels with NG-nitro-L-arginine methyl ester (200 microM), or removal of endothelium by mechanical rubbing, the relaxant responses were abolished. 3. The mean pEC50 values for relaxant responses followed the order: (+/-)-alpha-methyl-5-HT (8.80) > 5-HT (8.75) > ergotamine (8.17) > DHE (7.70) > 5-carboxamidotryptamine (7.62) > dihydroergocristine (7.17). 4. The relaxant effects of both ergotamine and dihydroergotamine were resistant to block by indomethacin (3 microM), prazosin (1 microM) and ketanserin (1 microM). However, the ergotamine-induced relaxation was highly susceptible to block by pizotifen (pA2 = 8.23), norclozapine (pA2 = 8.20), methiothepin (-log IC50 = 7.26), rauwolscine (pA2 = 7.24) and mesulergine (pA2 = 6.64). Each antagonist inhibited the relaxant responses to (+/-)-alpha-methyl-5-HT in the same manner with similar potency as that determined against ergotamine. 5. Recently, mRNA transcripts for 5-HT1D beta and 5-HT2B receptors have been demonstrated in porcine pulmonary arteries. The rank order of potencies of agonists and antagonists in the present study suggests that the relaxant responses to 5-HT and ergot derivatives are mediated through activation of endothelial 5-HT receptors which are similar to the 5-HT2B receptor subtypes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baxter G., Kennett G., Blaney F., Blackburn T. 5-HT2 receptor subtypes: a family re-united? Trends Pharmacol Sci. 1995 Mar;16(3):105–110. doi: 10.1016/s0165-6147(00)88991-9. [DOI] [PubMed] [Google Scholar]
- Bodelsson M., Törnebrandt K., Arneklo-Nobin B. Endothelial relaxing 5-hydroxytryptamine receptors in the rat jugular vein: similarity with the 5-hydroxytryptamine1C receptor. J Pharmacol Exp Ther. 1993 Feb;264(2):709–716. [PubMed] [Google Scholar]
- Bonhaus D. W., Bach C., DeSouza A., Salazar F. H., Matsuoka B. D., Zuppan P., Chan H. W., Eglen R. M. The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors. Br J Pharmacol. 1995 Jun;115(4):622–628. doi: 10.1111/j.1476-5381.1995.tb14977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Patch T. L., Kaumann A. J. The antimigraine drugs ergotamine and dihydroergotamine are potent 5-HT1C receptor agonists in piglet choroid plexus. Br J Pharmacol. 1991 Sep;104(1):45–48. doi: 10.1111/j.1476-5381.1991.tb12382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. S., Birraux G., Launay J. M., Maroteaux L. The human serotonin 5-HT2B receptor: pharmacological link between 5-HT2 and 5-HT1D receptors. FEBS Lett. 1994 Oct 3;352(3):393–399. doi: 10.1016/0014-5793(94)00968-6. [DOI] [PubMed] [Google Scholar]
- Ellis E. S., Byrne C., Murphy O. E., Tilford N. S., Baxter G. S. Mediation by 5-hydroxytryptamine2B receptors of endothelium-dependent relaxation in rat jugular vein. Br J Pharmacol. 1995 Jan;114(2):400–404. doi: 10.1111/j.1476-5381.1995.tb13240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari M. D., Saxena P. R. On serotonin and migraine: a clinical and pharmacological review. Cephalalgia. 1993 Jun;13(3):151–165. doi: 10.1046/j.1468-2982.1993.1303151.x. [DOI] [PubMed] [Google Scholar]
- Foguet M., Hoyer D., Pardo L. A., Parekh A., Kluxen F. W., Kalkman H. O., Stühmer W., Lübbert H. Cloning and functional characterization of the rat stomach fundus serotonin receptor. EMBO J. 1992 Sep;11(9):3481–3487. doi: 10.1002/j.1460-2075.1992.tb05427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozard J. R., Kalkman H. O. 5-Hydroxytryptamine (5-HT) and the initiation of migraine: new perspectives. Naunyn Schmiedebergs Arch Pharmacol. 1994 Sep;350(3):225–229. doi: 10.1007/BF00175026. [DOI] [PubMed] [Google Scholar]
- Glusa E., Markwardt F. Studies on 5-hydroxytryptamine receptors on isolated human femoral veins and arteries and the influence of dihydroergotamine. Pharmacology. 1984;29(6):336–342. doi: 10.1159/000138033. [DOI] [PubMed] [Google Scholar]
- Glusa E., Richter M. Endothelium-dependent relaxation of porcine pulmonary arteries via 5-HT1C-like receptors. Naunyn Schmiedebergs Arch Pharmacol. 1993 May;347(5):471–477. doi: 10.1007/BF00166737. [DOI] [PubMed] [Google Scholar]
- Hoyer D., Clarke D. E., Fozard J. R., Hartig P. R., Martin G. R., Mylecharane E. J., Saxena P. R., Humphrey P. P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev. 1994 Jun;46(2):157–203. [PubMed] [Google Scholar]
- Kalkman H. O. Is migraine prophylactic activity caused by 5-HT2B or 5-HT2C receptor blockade? Life Sci. 1994;54(10):641–644. doi: 10.1016/0024-3205(94)00546-x. [DOI] [PubMed] [Google Scholar]
- Kuoppamäki M., Syvälahti E., Hietala J. Clozapine and N-desmethylclozapine are potent 5-HT1C receptor antagonists. Eur J Pharmacol. 1993 Apr 15;245(2):179–182. doi: 10.1016/0922-4106(93)90126-t. [DOI] [PubMed] [Google Scholar]
- Martin G. R. Vascular receptors for 5-hydroxytryptamine: distribution, function and classification. Pharmacol Ther. 1994;62(3):283–324. doi: 10.1016/0163-7258(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Molderings G. J., Engel G., Roth E., Göthert M. Characterization of an endothelial 5-hydroxytryptamine (5-HT) receptor mediating relaxation of the porcine coronary artery. Naunyn Schmiedebergs Arch Pharmacol. 1989 Sep;340(3):300–308. doi: 10.1007/BF00168514. [DOI] [PubMed] [Google Scholar]
- Müller-Schweinitzer E. Alpha-adrenoceptors, 5-hydroxytryptamine receptors and the action of dihydroergotamine in human venous preparations obtained during saphenectomy procedures for varicose veins. Naunyn Schmiedebergs Arch Pharmacol. 1984 Oct;327(4):299–303. doi: 10.1007/BF00506240. [DOI] [PubMed] [Google Scholar]
- Müller-Schweinitzer E. Venoconstrictor responses to dihydroergocristine and dihydroergotamine: evidence for the involvement of 5-HT1 like receptors. Cardiovasc Drugs Ther. 1990 Dec;4(6):1455–1460. doi: 10.1007/BF02026491. [DOI] [PubMed] [Google Scholar]
- Müller H., Glusa E., Markwardt F. Dual effect of dihydroergotamine at vascular 5-hydroxytryptamine receptors in pithed rats. Pharmacology. 1988;37(4):248–253. doi: 10.1159/000138473. [DOI] [PubMed] [Google Scholar]
- Olesen J., Thomsen L. L., Iversen H. Nitric oxide is a key molecule in migraine and other vascular headaches. Trends Pharmacol Sci. 1994 May;15(5):149–153. doi: 10.1016/0165-6147(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J. Pharmacological differentiation of human 5-HT1B and 5-HT1D receptors. Biol Signals. 1994 Sep-Oct;3(5):217–222. [PubMed] [Google Scholar]
- Schmuck K., Ullmer C., Engels P., Lübbert H. Cloning and functional characterization of the human 5-HT2B serotonin receptor. FEBS Lett. 1994 Mar 28;342(1):85–90. doi: 10.1016/0014-5793(94)80590-3. [DOI] [PubMed] [Google Scholar]
- Schoeffter P., Hoyer D. 5-Hydroxytryptamine (5-HT)-induced endothelium-dependent relaxation of pig coronary arteries is mediated by 5-HT receptors similar to the 5-HT1D receptor subtype. J Pharmacol Exp Ther. 1990 Jan;252(1):387–395. [PubMed] [Google Scholar]
- Ullmer C., Schmuck K., Kalkman H. O., Lübbert H. Expression of serotonin receptor mRNAs in blood vessels. FEBS Lett. 1995 Aug 21;370(3):215–221. doi: 10.1016/0014-5793(95)00828-w. [DOI] [PubMed] [Google Scholar]
- VAN ROSSUM J. M. Cumulative dose-response curves. II. Technique for the making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch Int Pharmacodyn Ther. 1963;143:299–330. [PubMed] [Google Scholar]


