Abstract
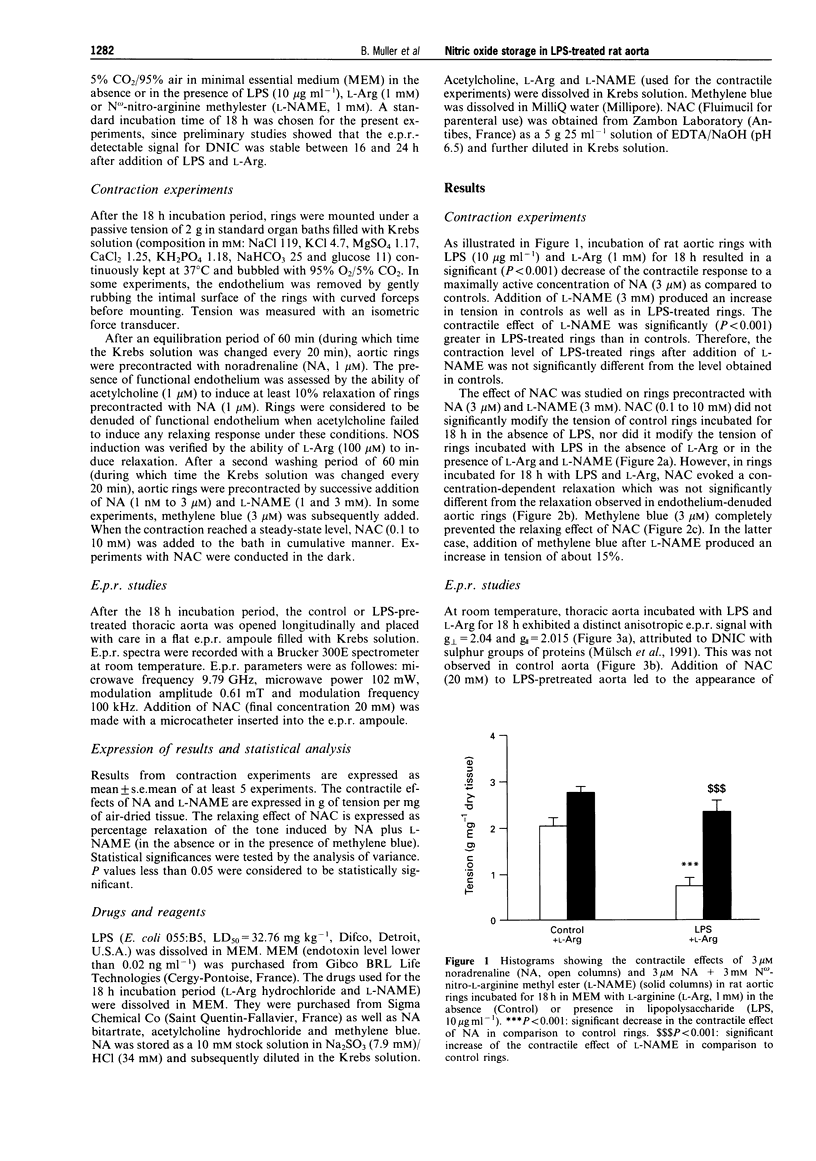
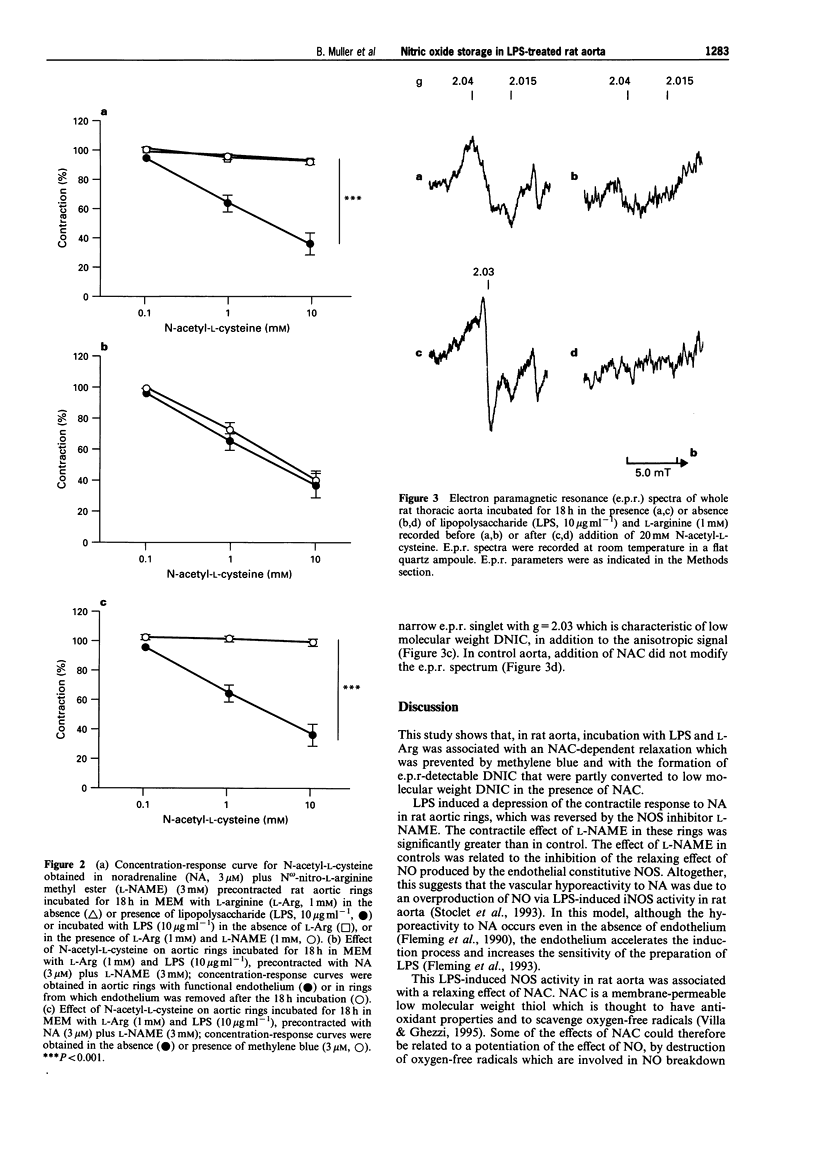
1. The aim of this study was to assess whether or not vasoactive nitric oxide (NO) stores exist within vascular tissue after lipopolysaccharide (LPS)-treatment. 2. Rat thoracic aortic rings (for contraction experiments) or whole thoracic aortae (for electron paramagnetic resonance (e.p.r.) spectroscopy) were incubated for 18 h at 37 degrees C in the absence (control) or in the presence of LPS (10 micrograms ml-1), with or without L-arginine (L-Arg, 1 mM), the substrate of NO synthase (NOS) or N omega-nitro-L-arginine methyl ester (L-NAME, 1 mM), an inhibitor of NOS. 3. Incubation of rat aortic rings with LPS and L-Arg resulted in a significant decrease of the maximum contractile response to noradrenaline (NA, 3 microM). Addition of L-NAME (3 mM) enhanced contraction towards control values. After precontraction with NA and L-NAME, addition of N-acetyl-L-cysteine (NAC, 0.1 to 10 mM) evoked a concentration-dependent relaxation in rings incubated with LPS and L-Arg, but not in control rings, rings incubated with LPS in the absence of L-Arg or rings incubated with LPS in the presence of L-Arg and L-NAME. Removal of the endothelium did not significantly modify the relaxation induced by NAC. Methylene blue (3 microM), an inhibitor of the activation of guanylyl cyclase by NO, completely abolished the relaxing effect of NAC. 4. The presence of protein-bound dinitrosyl non-haem iron complexes (DNIC) was detected by e.p.r. spectroscopy in aortae incubated with LPS and L-Arg, but not in control aortae. Furthermore in LPS-treated aortae, addition of NAC (20 mM) gave rise to the appearance of an e.p.r. signal characteristic of low molecular weight DNIC. 5. These results provide evidence that, within vascular tissue, NO generated from L-Arg by LPS-induced NOS activity can be stored as protein-bound DNIC in non-endothelial cells. Upon addition of NAC, low molecular weight DNIC are released from these storage sites and induce vascular relaxation probably through guanylyl cyclase activation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler A. R., Flitney F. W., Williams D. L. NO, nitrosonium ions, nitroxide ions, nitrosothiols and iron-nitrosyls in biology: a chemist's perspective. Trends Pharmacol Sci. 1995 Jan;16(1):18–22. doi: 10.1016/s0165-6147(00)88968-3. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., Lancaster J. R., Jr, Sweetland M. A., McDaniel M. L. Interleukin-1 beta-induced formation of EPR-detectable iron-nitrosyl complexes in islets of Langerhans. Role of nitric oxide in interleukin-1 beta-induced inhibition of insulin secretion. J Biol Chem. 1991 Nov 15;266(32):21351–21354. [PubMed] [Google Scholar]
- Drapier J. C., Pellat C., Henry Y. Generation of EPR-detectable nitrosyl-iron complexes in tumor target cells cocultured with activated macrophages. J Biol Chem. 1991 Jun 5;266(16):10162–10167. [PubMed] [Google Scholar]
- Fleming I., Gray G. A., Julou-Schaeffer G., Parratt J. R., Stoclet J. C. Incubation with endotoxin activates the L-arginine pathway in vascular tissue. Biochem Biophys Res Commun. 1990 Sep 14;171(2):562–568. doi: 10.1016/0006-291x(90)91183-s. [DOI] [PubMed] [Google Scholar]
- Fleming I., Gray G. A., Stoclet J. C. Influence of endothelium on induction of the L-arginine-nitric oxide pathway in rat aortas. Am J Physiol. 1993 Apr;264(4 Pt 2):H1200–H1207. doi: 10.1152/ajpheart.1993.264.4.H1200. [DOI] [PubMed] [Google Scholar]
- Hecker M., Boese M., Schini-Kerth V. B., Mülsch A., Busse R. Characterization of the stable L-arginine-derived relaxing factor released from cytokine-stimulated vascular smooth muscle cells as an NG-hydroxyl-L-arginine-nitric oxide adduct. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4671–4675. doi: 10.1073/pnas.92.10.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry Y., Lepoivre M., Drapier J. C., Ducrocq C., Boucher J. L., Guissani A. EPR characterization of molecular targets for NO in mammalian cells and organelles. FASEB J. 1993 Sep;7(12):1124–1134. doi: 10.1096/fasebj.7.12.8397130. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Lippton H., Edwards J. C., Baricos W. H., Hyman A. L., Kadowitz P. J., Gruetter C. A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981 Sep;218(3):739–749. [PubMed] [Google Scholar]
- Ignarro L. J. Signal transduction mechanisms involving nitric oxide. Biochem Pharmacol. 1991 Feb 15;41(4):485–490. doi: 10.1016/0006-2952(91)90618-f. [DOI] [PubMed] [Google Scholar]
- Julou-Schaeffer G., Gray G. A., Fleming I., Schott C., Parratt J. R., Stoclet J. C. Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am J Physiol. 1990 Oct;259(4 Pt 2):H1038–H1043. doi: 10.1152/ajpheart.1990.259.4.H1038. [DOI] [PubMed] [Google Scholar]
- Kennedy M. C., Gan T., Antholine W. E., Petering D. H. Metallothionein reacts with Fe2+ and NO to form products with A g = 2.039 ESR signal. Biochem Biophys Res Commun. 1993 Oct 29;196(2):632–635. doi: 10.1006/bbrc.1993.2296. [DOI] [PubMed] [Google Scholar]
- Lancaster J. R., Jr, Hibbs J. B., Jr EPR demonstration of iron-nitrosyl complex formation by cytotoxic activated macrophages. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1223–1227. doi: 10.1073/pnas.87.3.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Higgs E. A. Molecular mechanisms and therapeutic strategies related to nitric oxide. FASEB J. 1995 Oct;9(13):1319–1330. [PubMed] [Google Scholar]
- Moro M. A., Darley-Usmar V. M., Goodwin D. A., Read N. G., Zamora-Pino R., Feelisch M., Radomski M. W., Moncada S. Paradoxical fate and biological action of peroxynitrite on human platelets. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6702–6706. doi: 10.1073/pnas.91.14.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro M. A., Darley-Usmar V. M., Lizasoain I., Su Y., Knowles R. G., Radomski M. W., Moncada S. The formation of nitric oxide donors from peroxynitrite. Br J Pharmacol. 1995 Oct;116(3):1999–2004. doi: 10.1111/j.1476-5381.1995.tb16404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mülsch A., Mordvintcev P., Vanin A. F., Busse R. The potent vasodilating and guanylyl cyclase activating dinitrosyl-iron(II) complex is stored in a protein-bound form in vascular tissue and is released by thiols. FEBS Lett. 1991 Dec 9;294(3):252–256. doi: 10.1016/0014-5793(91)81441-a. [DOI] [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Nüssler A. K., Geller D. A., Sweetland M. A., Di Silvio M., Billiar T. R., Madariaga J. B., Simmons R. L., Lancaster J. R., Jr Induction of nitric oxide synthesis and its reactions in cultured human and rat hepatocytes stimulated with cytokines plus LPS. Biochem Biophys Res Commun. 1993 Jul 30;194(2):826–835. doi: 10.1006/bbrc.1993.1896. [DOI] [PubMed] [Google Scholar]
- Pellat C., Henry Y., Drapier J. C. IFN-gamma-activated macrophages: detection by electron paramagnetic resonance of complexes between L-arginine-derived nitric oxide and non-heme iron proteins. Biochem Biophys Res Commun. 1990 Jan 15;166(1):119–125. doi: 10.1016/0006-291x(90)91919-j. [DOI] [PubMed] [Google Scholar]
- Schwarz M. A., Lazo J. S., Yalowich J. C., Allen W. P., Whitmore M., Bergonia H. A., Tzeng E., Billiar T. R., Robbins P. D., Lancaster J. R., Jr Metallothionein protects against the cytotoxic and DNA-damaging effects of nitric oxide. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4452–4456. doi: 10.1073/pnas.92.10.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler J., Bergonia H. A., Di Silvio M., Sweetland M. A., Billiar T. R., Simmons R. L., Lancaster J. R., Jr Nonheme iron-nitrosyl complex formation in rat hepatocytes: detection by electron paramagnetic resonance spectroscopy. Arch Biochem Biophys. 1993 Apr;302(1):4–11. doi: 10.1006/abbi.1993.1173. [DOI] [PubMed] [Google Scholar]
- Stamler J. S. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994 Sep 23;78(6):931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Vanin A. F. Endothelium-derived relaxing factor is a nitrosyl iron complex with thiol ligands. FEBS Lett. 1991 Sep 2;289(1):1–3. doi: 10.1016/0014-5793(91)80894-9. [DOI] [PubMed] [Google Scholar]
- Vanin A. F., Kiladze S. V., Kubrina L. N. O vkliuchenii nizkomolekuliarnykh SH-soderzhashchikh soedinenii v nitrozil'nye kompleksy negemovogo zheleza v beskletochnykh i kletochnykh preparatakh. Biofizika. 1975 Nov-Dec;20(6):1068–1072. [PubMed] [Google Scholar]
- Vanin A. F., Men'shikov G. B., Moroz I. A., Mordvintcev P. I., Serezhenkov V. A., Burbaev D. Sh. The source of non-heme iron that binds nitric oxide in cultivated macrophages. Biochim Biophys Acta. 1992 Jun 29;1135(3):275–279. doi: 10.1016/0167-4889(92)90231-y. [DOI] [PubMed] [Google Scholar]
- Vedernikov Y. P., Mordvintcev P. I., Malenkova I. V., Vanin A. F. Similarity between the vasorelaxing activity of dinitrosyl iron cysteine complexes and endothelium-derived relaxing factor. Eur J Pharmacol. 1992 Feb 18;211(3):313–317. doi: 10.1016/0014-2999(92)90386-i. [DOI] [PubMed] [Google Scholar]
- Villa P., Ghezzi P. Effect of N-acetyl-L-cysteine on sepsis in mice. Eur J Pharmacol. 1995 Mar 16;292(3-4):341–344. doi: 10.1016/0926-6917(95)90043-8. [DOI] [PubMed] [Google Scholar]


