Abstract
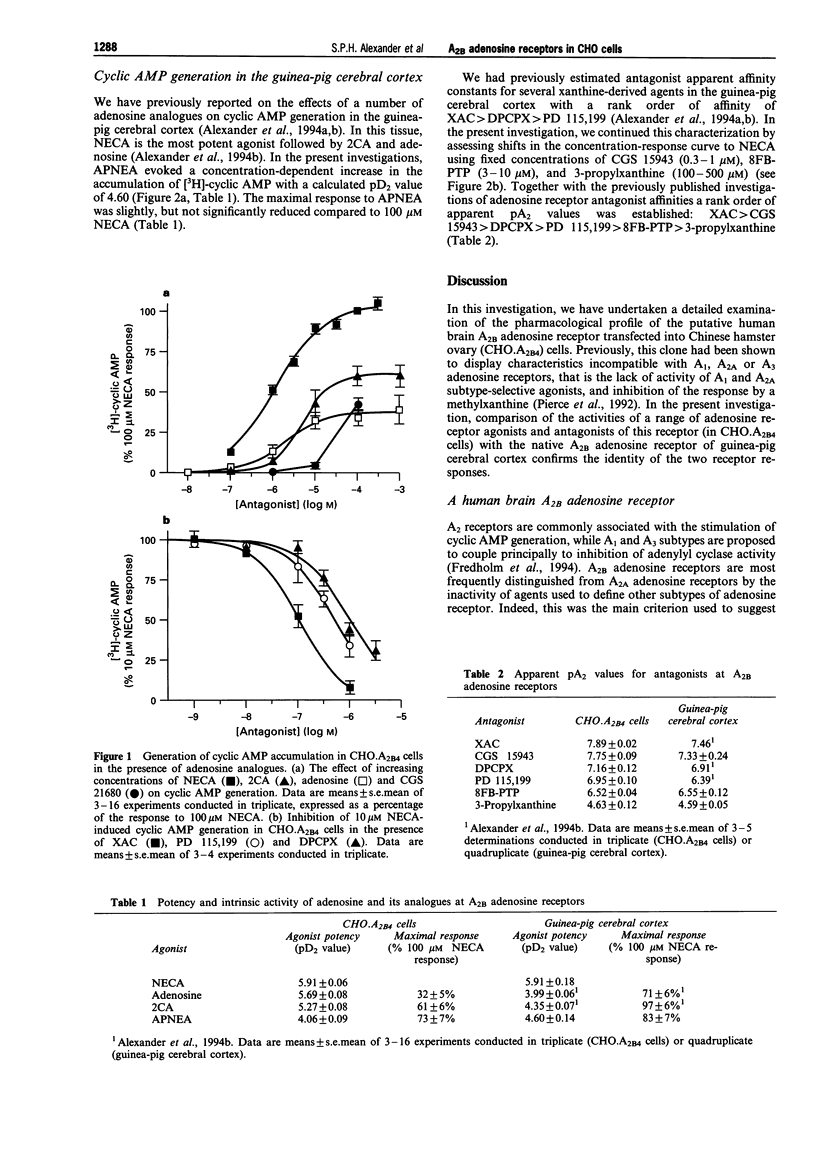
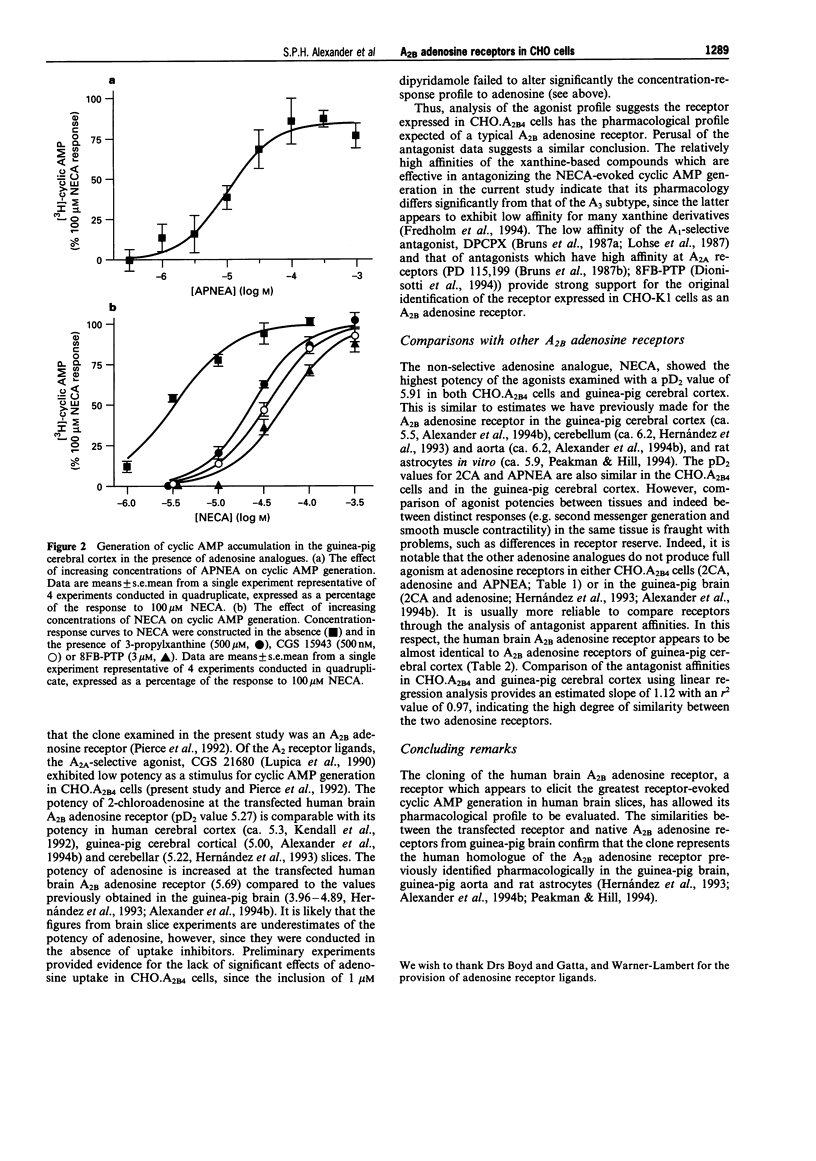
1. An [3H]-adenine pre-labelling methodology was employed to assay cyclic AMP generation by adenosine analogues in Chinese hamster ovary (CHO.A2B4) cells, transfected with cDNA which has been proposed to code for the human brain A2B adenosine receptor, and in guinea-pig cerebral cortical slices. 2. Adenosine analogues showing the following rank order of potency in the CHO.A2B4 cells (pD2 value): 5'-N-ethylcarboxamidoadenosine (NECA, 5.91) > adenosine (5.69) > 2-chloroadenosine (5.27) > N6-(2-(4-aminophenyl)-ethylamino)adenosine (APNEA, 4.06). The purportedly A2A-selective agonist, CGS 21680, failed to elicit a significant stimulation of cyclic AMP generation at concentrations up to 10 microM in CHO.A2B4 cells. In the guinea-pig cerebral cortex, NECA was more potent than APNEA with pD2 values of 5.91 and 4.60, respectively. 3. Of these agents, NECA was observed to exhibit the greatest intrinsic activity in CHO.A2B4 cells (ca. 10 fold stimulation of cyclic AMP), while, in comparison, maximal responses to adenosine (32% NECA response), 2-chloroadenosine (61%), and APNEA (73%) were reduced. 4. Antagonists of NECA-evoked cyclic AMP generation showed the rank order of apparent affinity (apparent pA2 value in CHO.A2B4 cells: guinea-pig cerebral cortex): XAC (7.89: 7.46) > CGS 15943 (7.75: 7.33) > DPCPX (7.16: 6.91) > PD 115,199 (6.95: 6.39) > 8FB-PTP (6.52: 6.55) > 3-propylxanthine (4.63: 4.59). 5. We conclude that, using the agents tested, the A2B adenosine receptor cloned from human brain expressed in Chinese hamster ovary cells exhibits an identical pharmacological profile to native A2B receptors in guinea-pig brain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander S. P., Curtis A. R., Kendall D. A., Hill S. J. A1 adenosine receptor inhibition of cyclic AMP formation and radioligand binding in the guinea-pig cerebral cortex. Br J Pharmacol. 1994 Dec;113(4):1501–1507. doi: 10.1111/j.1476-5381.1994.tb17166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander S. P., Losinski A., Kendall D. A., Hill S. J. A comparison of A2 adenosine receptor-induced cyclic AMP generation in cerebral cortex and relaxation of pre-contracted aorta. Br J Pharmacol. 1994 Jan;111(1):185–190. doi: 10.1111/j.1476-5381.1994.tb14042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns R. F., Fergus J. H., Badger E. W., Bristol J. A., Santay L. A., Hartman J. D., Hays S. J., Huang C. C. Binding of the A1-selective adenosine antagonist 8-cyclopentyl-1,3-dipropylxanthine to rat brain membranes. Naunyn Schmiedebergs Arch Pharmacol. 1987 Jan;335(1):59–63. doi: 10.1007/BF00165037. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Fergus J. H., Badger E. W., Bristol J. A., Santay L. A., Hays S. J. PD 115,199: an antagonist ligand for adenosine A2 receptors. Naunyn Schmiedebergs Arch Pharmacol. 1987 Jan;335(1):64–69. doi: 10.1007/BF00165038. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hill S. J., Alexander S. P., Rubin P. C., Horn E. H. Adenosine receptor-induced cyclic AMP generation and inhibition of 5-hydroxytryptamine release in human platelets. Br J Clin Pharmacol. 1995 Jul;40(1):43–50. doi: 10.1111/j.1365-2125.1995.tb04533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. W., Butts-Lamb P., Padgett W. Subclasses of adenosine receptors in the central nervous system: interaction with caffeine and related methylxanthines. Cell Mol Neurobiol. 1983 Mar;3(1):69–80. doi: 10.1007/BF00734999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisotti S., Conti A., Sandoli D., Zocchi C., Gatta F., Ongini E. Effects of the new A2 adenosine receptor antagonist 8FB-PTP, an 8 substituted pyrazolo-triazolo-pyrimidine, on in vitro functional models. Br J Pharmacol. 1994 Jun;112(2):659–665. doi: 10.1111/j.1476-5381.1994.tb13126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm B. B., Abbracchio M. P., Burnstock G., Daly J. W., Harden T. K., Jacobson K. A., Leff P., Williams M. Nomenclature and classification of purinoceptors. Pharmacol Rev. 1994 Jun;46(2):143–156. [PMC free article] [PubMed] [Google Scholar]
- Hernández F., Kendall D. A., Alexander S. P. Adenosine receptor-induced second messenger production in adult guinea-pig cerebellum. Br J Pharmacol. 1993 Nov;110(3):1085–1090. doi: 10.1111/j.1476-5381.1993.tb13925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall D. A., Millns P. J., Firth J. L. Direct and indirect stimulations of cyclic AMP formation in human brain. Br J Pharmacol. 1992 Apr;105(4):899–902. doi: 10.1111/j.1476-5381.1992.tb09075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse M. J., Klotz K. N., Lindenborn-Fotinos J., Reddington M., Schwabe U., Olsson R. A. 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX)--a selective high affinity antagonist radioligand for A1 adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1987 Aug;336(2):204–210. doi: 10.1007/BF00165806. [DOI] [PubMed] [Google Scholar]
- Losinski A., Alexander S. P. Adenosine receptor-mediated relaxation of guinea-pig precontracted, isolated trachea. Br J Pharmacol. 1995 Nov;116(5):2425–2428. doi: 10.1111/j.1476-5381.1995.tb15090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica C. R., Cass W. A., Zahniser N. R., Dunwiddie T. V. Effects of the selective adenosine A2 receptor agonist CGS 21680 on in vitro electrophysiology, cAMP formation and dopamine release in rat hippocampus and striatum. J Pharmacol Exp Ther. 1990 Mar;252(3):1134–1141. [PubMed] [Google Scholar]
- Peakman M. C., Hill S. J. Adenosine A2B-receptor-mediated cyclic AMP accumulation in primary rat astrocytes. Br J Pharmacol. 1994 Jan;111(1):191–198. doi: 10.1111/j.1476-5381.1994.tb14043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K. D., Furlong T. J., Selbie L. A., Shine J. Molecular cloning and expression of an adenosine A2b receptor from human brain. Biochem Biophys Res Commun. 1992 Aug 31;187(1):86–93. doi: 10.1016/s0006-291x(05)81462-7. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]


