Abstract
1. Simultaneous recordings of tension and [Ca2+]i were made in rat anococcygeus muscle strips to investigate possible mechanisms involved during cyclic nucleotide-mediated relaxation. Relaxation of pre-contracted muscles was induced by sodium nitroprusside (SNP) or forskolin and the effects of cyclopiazonic acid (CPA) on these responses were examined. 2. In muscles pre-contracted with 0.2 microM phenylephrine addition of SNP (10 microM) caused a rapid and near complete relaxation of force. This was accompanied by a decrease in [Ca2+]i, however, this was not of a comparable magnitude to the decrease in force. The level of [Ca2+]i in muscles relaxed with SNP was shown to be associated with substantially higher force levels in the absence of SNP. Forskolin (10 microM) caused a slower, essentially complete relaxation which was associated with a proportional decrease in [Ca2+]i. 3. In muscles pretreated with SNP or forskolin subsequent responses to phenylephrine were attenuated with both force and [Ca2+]i rising slowly to attain eventually levels similar to those observed when the relaxant was applied to pre-contracted muscles. 4. Exposure of the muscles to the sarcoplasmic reticulum Ca(2+)-ATPase inhibitor, CPA (10 microM), resulted in a sustained increase in [Ca2+]i which, in most cases, was not associated with any force development. The relaxation and decrease in [Ca2+]i in response to both SNP and forskolin were attenuated and substantially slowed in the presence of CPA. Overall the extent of this attenuation was greater for SNP. For both SNP and forskolin, CPA attenuated the decrease in [Ca2+]i to a greater extent than the decrease in force. In some cases, SNP-mediated relaxation in the presence of CPA was observed with almost no detectable change in [Ca2+]i. 5. The results suggest that, in the rat anococcygeus muscle under normal circumstances, a lowering of [Ca2+]i can fully account for the relaxation induced by forskolin but not for that induced by SNP, where mechanisms independent of changes in [Ca2+]i appear to contribute. Whilst Ca2+ sequestration into the sarcoplasmic reticulum plays a role in the relaxation mediated by both SNP and forskolin other Ca2+ lowering mechanisms may also be involved, especially in the response to forskolin.
Full text
PDF

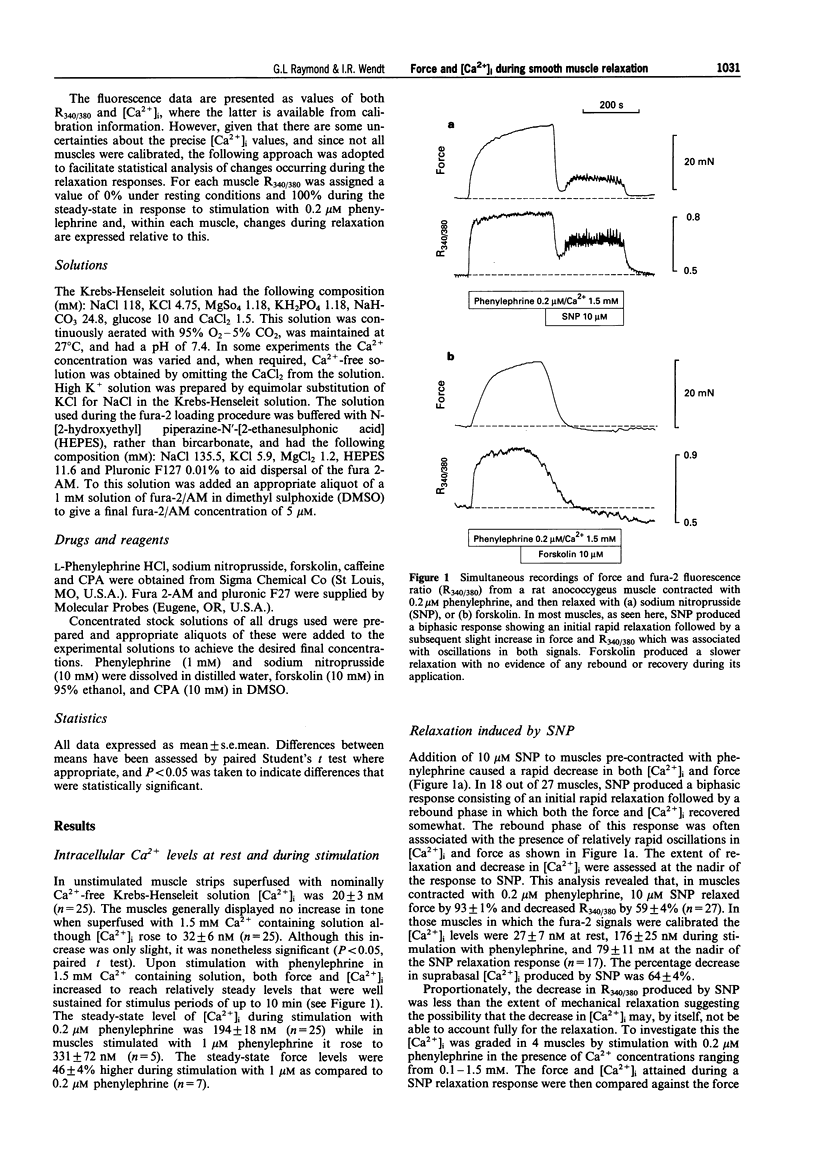
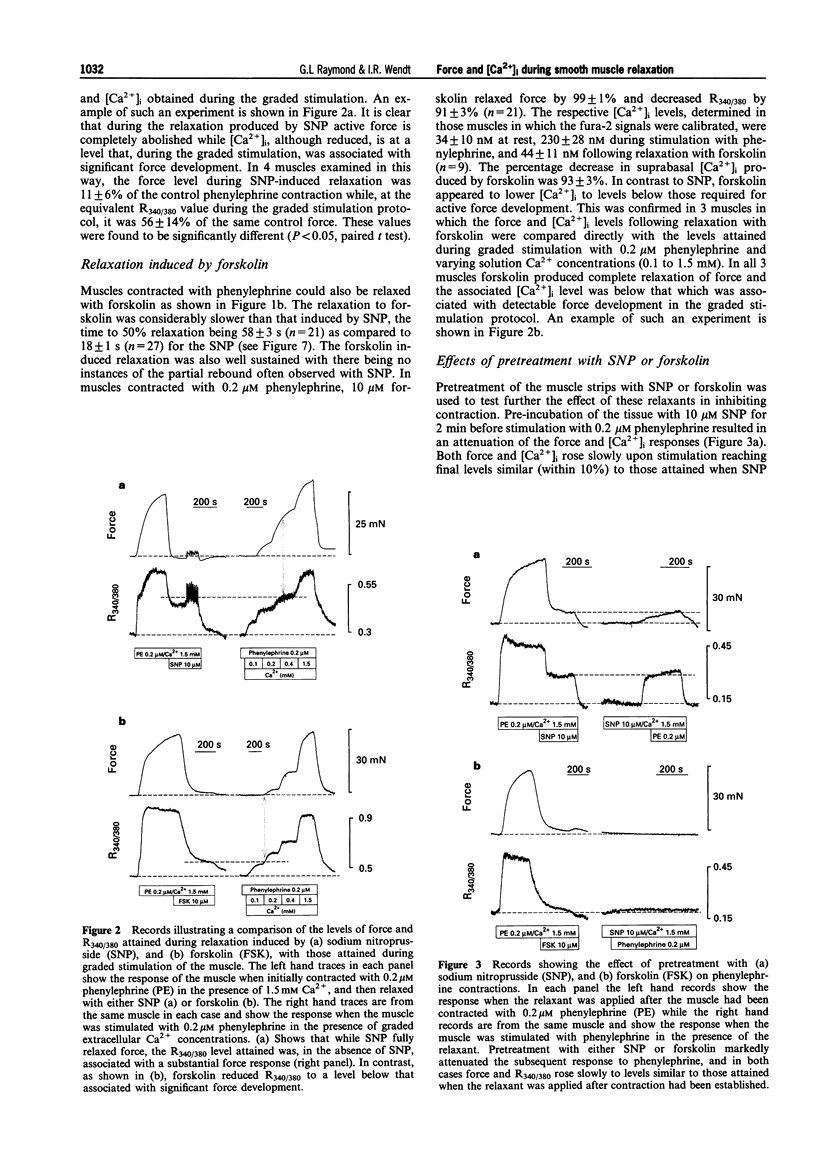
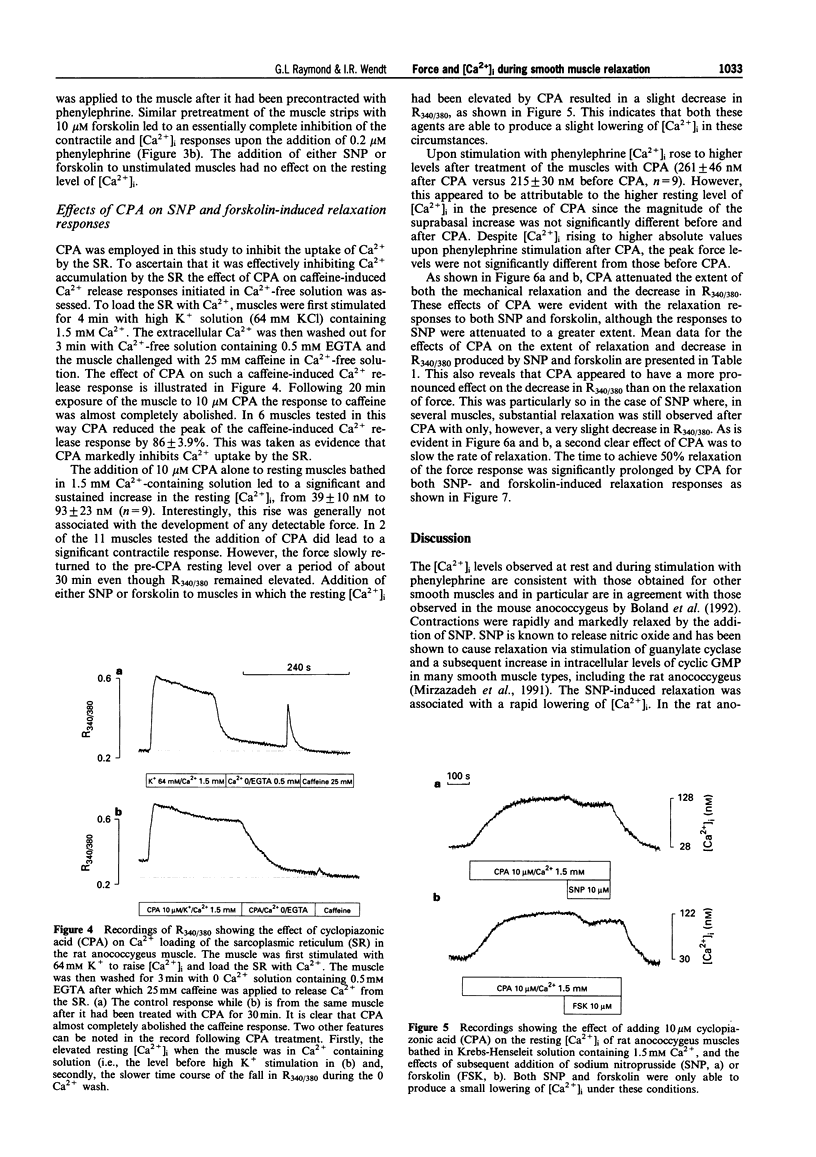
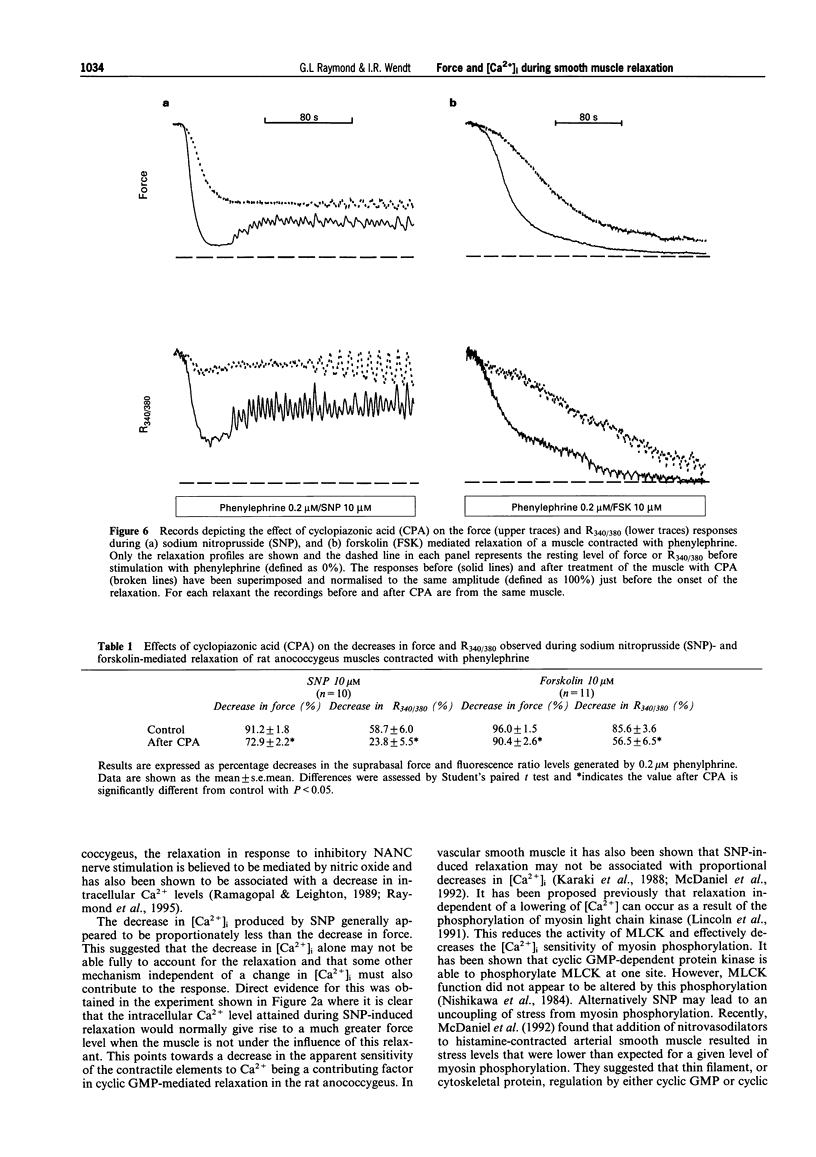
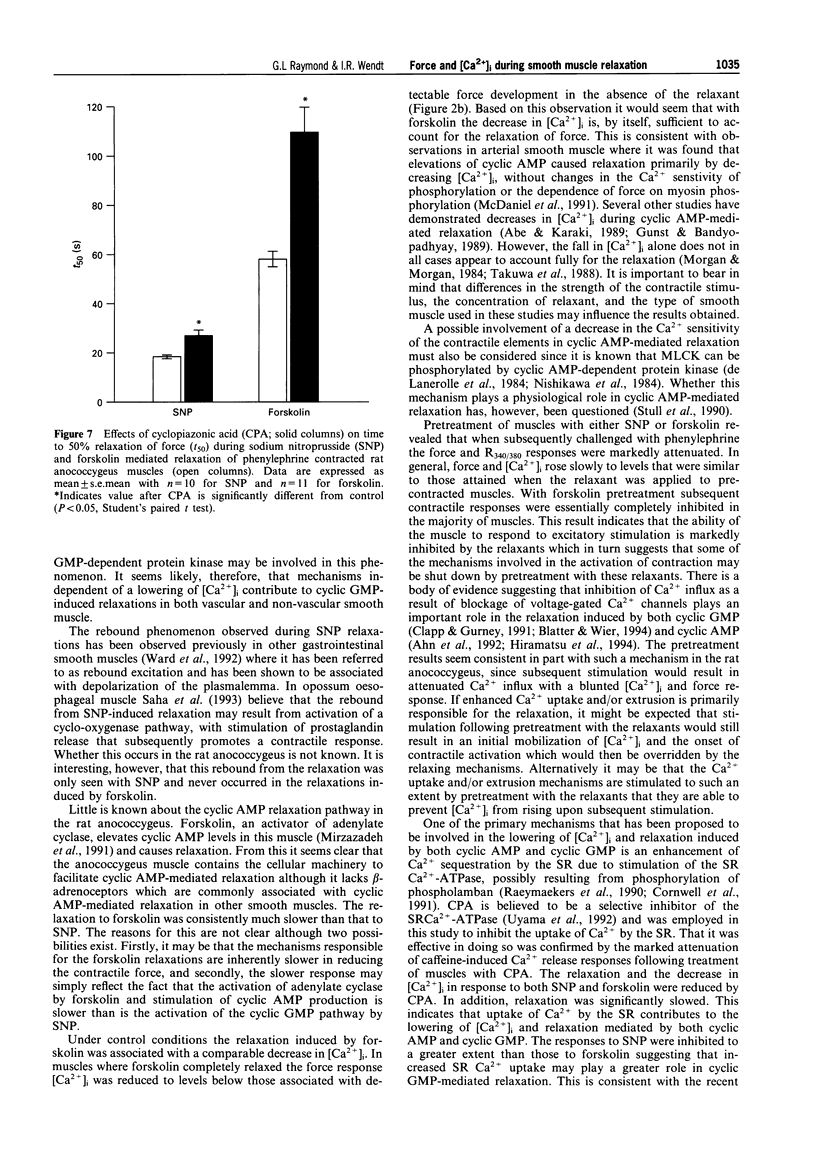


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe A., Karaki H. Effect of forskolin on cytosolic Ca++ level and contraction in vascular smooth muscle. J Pharmacol Exp Ther. 1989 Jun;249(3):895–900. [PubMed] [Google Scholar]
- Ahn H. Y., Kang S. E., Chang K. C., Karaki H. Dibutyryl cyclic AMP and forskolin inhibit phosphatidylinositol hydrolysis, Ca2+ influx and contraction in vascular smooth muscle. Jpn J Pharmacol. 1992 Jun;59(2):263–265. doi: 10.1254/jjp.59.263. [DOI] [PubMed] [Google Scholar]
- Blatter L. A., Wier W. G. Nitric oxide decreases [Ca2+]i in vascular smooth muscle by inhibition of the calcium current. Cell Calcium. 1994 Feb;15(2):122–131. doi: 10.1016/0143-4160(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Boland B., Himpens B., Gillis J. M., Casteels R. Relationship between force and Ca2+ in anococcygeal and vas deferens smooth muscle cells of the mouse. Pflugers Arch. 1992 May;421(1):43–51. doi: 10.1007/BF00374732. [DOI] [PubMed] [Google Scholar]
- Chen Q., van Breemen C. The superficial buffer barrier in venous smooth muscle: sarcoplasmic reticulum refilling and unloading. Br J Pharmacol. 1993 Jun;109(2):336–343. doi: 10.1111/j.1476-5381.1993.tb13575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp L. H., Gurney A. M. Modulation of calcium movements by nitroprusside in isolated vascular smooth muscle cells. Pflugers Arch. 1991 Jun;418(5):462–470. doi: 10.1007/BF00497774. [DOI] [PubMed] [Google Scholar]
- Cornwell T. L., Pryzwansky K. B., Wyatt T. A., Lincoln T. M. Regulation of sarcoplasmic reticulum protein phosphorylation by localized cyclic GMP-dependent protein kinase in vascular smooth muscle cells. Mol Pharmacol. 1991 Dec;40(6):923–931. [PubMed] [Google Scholar]
- Crichton C. A., Smith G. L., Miller D. J., McGrath J. C. The modulation of force in isolated rat EGTA-treated anococcygeus muscle by phosphate, cyclic AMP and noradrenaline. Q J Exp Physiol. 1989 Nov;74(6):943–945. doi: 10.1113/expphysiol.1989.sp003365. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Tawada Y., Shigekawa M. Regulation of the plasma membrane Ca2+ pump by cyclic nucleotides in cultured vascular smooth muscle cells. J Biol Chem. 1988 Jun 15;263(17):8058–8065. [PubMed] [Google Scholar]
- Gibson A., Brave S. R., McFadzean I., Mirzazadeh S., Tucker J. F., Wayman C. Nitrergic stimulation does not inhibit carbachol-induced inositol phosphate generation in the rat anococcygeus. Neurosci Lett. 1994 Aug 29;178(1):35–38. doi: 10.1016/0304-3940(94)90283-6. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Gunst S. J., Bandyopadhyay S. Contractile force and intracellular Ca2+ during relaxation of canine tracheal smooth muscle. Am J Physiol. 1989 Aug;257(2 Pt 1):C355–C364. doi: 10.1152/ajpcell.1989.257.2.C355. [DOI] [PubMed] [Google Scholar]
- Hiramatsu T., Kume H., Kotlikoff M. I., Takagi K. Role of calcium-activated potassium channels in the relaxation of tracheal smooth muscles by forskolin. Clin Exp Pharmacol Physiol. 1994 May;21(5):367–375. doi: 10.1111/j.1440-1681.1994.tb02529.x. [DOI] [PubMed] [Google Scholar]
- Hirata M., Kohse K. P., Chang C. H., Ikebe T., Murad F. Mechanism of cyclic GMP inhibition of inositol phosphate formation in rat aorta segments and cultured bovine aortic smooth muscle cells. J Biol Chem. 1990 Jan 25;265(3):1268–1273. [PubMed] [Google Scholar]
- Ito S., Suzuki S., Itoh T. Effects of a water-soluble forskolin derivative (NKH477) and a membrane-permeable cyclic AMP analogue on noradrenaline-induced Ca2+ mobilization in smooth muscle of rabbit mesenteric artery. Br J Pharmacol. 1993 Nov;110(3):1117–1125. doi: 10.1111/j.1476-5381.1993.tb13930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juneja R., Ito E., Koide S. S. Effect of serotonin and tricyclic antidepressants on intracellular calcium concentrations in Spisula oocytes. Cell Calcium. 1994 Jan;15(1):1–6. doi: 10.1016/0143-4160(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Karaki H., Sato K., Ozaki H., Murakami K. Effects of sodium nitroprusside on cytosolic calcium level in vascular smooth muscle. Eur J Pharmacol. 1988 Nov 1;156(2):259–266. doi: 10.1016/0014-2999(88)90329-9. [DOI] [PubMed] [Google Scholar]
- Li C. G., Rand M. J. Evidence for a role of nitric oxide in the neurotransmitter system mediating relaxation of the rat anococcygeus muscle. Clin Exp Pharmacol Physiol. 1989 Dec;16(12):933–938. doi: 10.1111/j.1440-1681.1989.tb02404.x. [DOI] [PubMed] [Google Scholar]
- Lincoln T. M., Cornwell T. L., Rashatwar S. S., Johnson R. M. Mechanism of cyclic-GMP-dependent relaxation in vascular smooth muscle. Biochem Soc Trans. 1988 Aug;16(4):497–499. doi: 10.1042/bst0160497. [DOI] [PubMed] [Google Scholar]
- Lincoln T. M., Cornwell T. L., Taylor A. E. cGMP-dependent protein kinase mediates the reduction of Ca2+ by cAMP in vascular smooth muscle cells. Am J Physiol. 1990 Mar;258(3 Pt 1):C399–C407. doi: 10.1152/ajpcell.1990.258.3.C399. [DOI] [PubMed] [Google Scholar]
- Luo D. L., Nakazawa M., Ishibashi T., Kato K., Imai S. Putative, selective inhibitors of sarcoplasmic reticulum Ca+(+)-pump ATPase inhibit relaxation by nitroglycerin and atrial natriuretic factor of the rabbit aorta contracted by phenylephrine. J Pharmacol Exp Ther. 1993 Jun;265(3):1187–1192. [PubMed] [Google Scholar]
- McDaniel N. L., Chen X. L., Singer H. A., Murphy R. A., Rembold C. M. Nitrovasodilators relax arterial smooth muscle by decreasing [Ca2+]i and uncoupling stress from myosin phosphorylation. Am J Physiol. 1992 Aug;263(2 Pt 1):C461–C467. doi: 10.1152/ajpcell.1992.263.2.C461. [DOI] [PubMed] [Google Scholar]
- McDaniel N. L., Rembold C. M., Richard H. M., Murphy R. A. Cyclic AMP relaxes swine arterial smooth muscle predominantly by decreasing cell Ca2+ concentration. J Physiol. 1991 Aug;439:147–160. doi: 10.1113/jphysiol.1991.sp018661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrogan I., Lu S., Hipworth S., Sormaz L., Eng R., Preocanin D., Daniel E. E. Mechanisms of cyclic nucleotide-induced relaxation in canine tracheal smooth muscle. Am J Physiol. 1995 Mar;268(3 Pt 1):L407–L413. doi: 10.1152/ajplung.1995.268.3.L407. [DOI] [PubMed] [Google Scholar]
- Mirzazadeh S., Hobbs A. J., Tucker J. F., Gibson A. Cyclic nucleotide content of the rat anococcygeus during relaxations induced by drugs or by non-adrenergic, non-cholinergic field stimulation. J Pharm Pharmacol. 1991 Apr;43(4):247–251. doi: 10.1111/j.2042-7158.1991.tb06677.x. [DOI] [PubMed] [Google Scholar]
- Morgan J. P., Morgan K. G. Stimulus-specific patterns of intracellular calcium levels in smooth muscle of ferret portal vein. J Physiol. 1984 Jun;351:155–167. doi: 10.1113/jphysiol.1984.sp015239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy K. S., Severi C., Grider J. R., Makhlouf G. M. Inhibition of IP3 and IP3-dependent Ca2+ mobilization by cyclic nucleotides in isolated gastric muscle cells. Am J Physiol. 1993 May;264(5 Pt 1):G967–G974. doi: 10.1152/ajpgi.1993.264.5.G967. [DOI] [PubMed] [Google Scholar]
- Nishikawa M., de Lanerolle P., Lincoln T. M., Adelstein R. S. Phosphorylation of mammalian myosin light chain kinases by the catalytic subunit of cyclic AMP-dependent protein kinase and by cyclic GMP-dependent protein kinase. J Biol Chem. 1984 Jul 10;259(13):8429–8436. [PubMed] [Google Scholar]
- Putney J. W., Jr Capacitative calcium entry revisited. Cell Calcium. 1990 Nov-Dec;11(10):611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- Raeymaekers L., Eggermont J. A., Wuytack F., Casteels R. Effects of cyclic nucleotide dependent protein kinases on the endoplasmic reticulum Ca2+ pump of bovine pulmonary artery. Cell Calcium. 1990 Apr;11(4):261–268. doi: 10.1016/0143-4160(90)90002-c. [DOI] [PubMed] [Google Scholar]
- Ramagopal M. V., Leighton H. J. Effects of NG-monomethyl-L-arginine on field stimulation-induced decreases in cytosolic Ca2+ levels and relaxation in the rat anococcygeus muscle. Eur J Pharmacol. 1989 Dec 19;174(2-3):297–299. doi: 10.1016/0014-2999(89)90325-7. [DOI] [PubMed] [Google Scholar]
- Raymond G. L., Wendt I. R., Kotsanas G. Force and intracellular Ca2+ during NANC-mediated relaxation of rat anococcygeus muscle and the effects of cyclopiazonic acid. Clin Exp Pharmacol Physiol. 1995 Oct;22(10):717–723. doi: 10.1111/j.1440-1681.1995.tb01925.x. [DOI] [PubMed] [Google Scholar]
- Saha J. K., Hirano I., Goyal R. K. Biphasic effect of SNP on opossum esophageal longitudinal muscle: involvement of cGMP and eicosanoids. Am J Physiol. 1993 Aug;265(2 Pt 1):G403–G407. doi: 10.1152/ajpgi.1993.265.2.G403. [DOI] [PubMed] [Google Scholar]
- Stull J. T., Hsu L. C., Tansey M. G., Kamm K. E. Myosin light chain kinase phosphorylation in tracheal smooth muscle. J Biol Chem. 1990 Sep 25;265(27):16683–16690. [PubMed] [Google Scholar]
- Takuwa Y., Takuwa N., Rasmussen H. The effects of isoproterenol on intracellular calcium concentration. J Biol Chem. 1988 Jan 15;263(2):762–768. [PubMed] [Google Scholar]
- Uyama Y., Imaizumi Y., Watanabe M. Effects of cyclopiazonic acid, a novel Ca(2+)-ATPase inhibitor, on contractile responses in skinned ileal smooth muscle. Br J Pharmacol. 1992 May;106(1):208–214. doi: 10.1111/j.1476-5381.1992.tb14316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrolix M., Raeymaekers L., Wuytack F., Hofmann F., Casteels R. Cyclic GMP-dependent protein kinase stimulates the plasmalemmal Ca2+ pump of smooth muscle via phosphorylation of phosphatidylinositol. Biochem J. 1988 Nov 1;255(3):855–863. doi: 10.1042/bj2550855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S. M., Dalziel H. H., Thornbury K. D., Westfall D. P., Sanders K. M. Nonadrenergic, noncholinergic inhibition and rebound excitation in canine colon depend on nitric oxide. Am J Physiol. 1992 Feb;262(2 Pt 1):G237–G243. doi: 10.1152/ajpgi.1992.262.2.G237. [DOI] [PubMed] [Google Scholar]
- de Lanerolle P., Nishikawa M., Yost D. A., Adelstein R. S. Increased phosphorylation of myosin light chain kinase after an increase in cyclic AMP in intact smooth muscle. Science. 1984 Mar 30;223(4643):1415–1417. doi: 10.1126/science.6322302. [DOI] [PubMed] [Google Scholar]


